The International Olive Council (IOC) is currently inviting applications for scholarships related to the International Expert Course in Virgin Olive Oil Tasting.
The International Olive Council (IOC) is currently inviting applications for scholarships related to the International Expert Course in Virgin Olive Oil Tasting.
This educational opportunity is being hosted by the University of Jaén in Spain and aims to advance technology transfer to improve participants' tasting abilities. It focuses on imparting foundational knowledge about the sensory evaluation of virgin olive oil, its methodology, and the association between olive oil's organoleptic traits and its cultivation and extraction methods, alongside various physical and chemical quality control standards.
This call is open to professionals from countries that are members of the IOC, who have a minimum of five years' experience working in olive oil mills, quality control of olive oil, or in the research and development of olive oil. Applicants should also have a basic educational qualification. The course is scheduled to run from the 25th of September to the 19th of December, 2024, in Jaén, Spain.
Interested individuals must submit their scholarship applications by the 7th of April, 2024.
For additional details about the course, the scholarships offered by the IOC (including principles, exclusions, selection, and award criteria), and the application process, more information can be found HERE.
Attached for further reference is the brochure: Triptych_Olive_March_2024.
Source:
https://www.internationaloliveoil.org/applications-for-scholarships-for-the-international-expert-course-in-virgin-olive-oil-tasting/
Celebrate Olive Oil Excellence and Sustainability at the Final ARTOLIO ENI CBC MED Meeting in Barcelona!
 Save the Date: Celebrate Olive Oil Excellence and Sustainability at the Final ARTOLIO ENI CBC MED Meeting in Barcelona!
Save the Date: Celebrate Olive Oil Excellence and Sustainability at the Final ARTOLIO ENI CBC MED Meeting in Barcelona!

 What to Expect?
What to Expect?
 ARTOLIO Platform
ARTOLIO Platform
 Special Highlights
Special Highlights
 Agenda Snapshot
Agenda Snapshot
 Venue: Petit Palace Museum, Barcelona
Venue: Petit Palace Museum, Barcelona
 If you are a producer, book your place on our platform today and become part of the history of olive oil!
If you are a producer, book your place on our platform today and become part of the history of olive oil! 
AgroInfluencers: The Emerging Voices in the Agricultural Landscape
AgroInfluencers: The Emerging Voices in the Agricultural Landscape
In the digital age, 'influencers' are transforming every sector, from fashion and food to travel and fitness. In the agriculture sector, a new breed of influencers known as 'agroinfluencers' are stepping into the limelight. These modern farmers, agricultural students, and farming enthusiasts use social media to share their daily lives, insights, and knowledge about agriculture, inspire others, and reshape the perception of rural life.
1. Understanding AgroInfluencers
An agroinfluencer is an individual with the ability to influence others' perceptions, opinions, and decisions related to agriculture and rural life. They are typically young farmers, agricultural engineers, veterinarians, forestry workers, and other professionals in the agriculture sector. They leverage social media platforms to share their work and lifestyle in the countryside.
Their influence extends beyond their immediate followers. They engage with agricultural machinery companies, agricultural brands, and even government institutions. Their content ranges from practical farming tips, educational information about agricultural practices, to personal narratives about rural life.
1.1 The Rise of AgroInfluencers
The rise of agroinfluencers is largely attributed to the digital revolution and the growing popularity of social media platforms. As of January 2023, there were 5.16 billion internet users worldwide, representing 64.4% of the global population. Of these, 4.76 billion, or 59.4% of the global population, are social media users.
The proliferation of social media platforms has provided an avenue for individuals to share their thoughts, experiences, information, ideas, and opinions with a broad audience. This is particularly beneficial for individuals looking to establish their personal brand or business. Social media platforms offer a platform to reach a wider audience and build an online community.
In the agriculture sector, the use of social media has been on the rise. Farmers and agricultural professionals have discovered the numerous benefits these platforms offer, such as connecting with other farmers and promoting their products.
2. The Role of AgroInfluencers
Agroinfluencers play a crucial role in the agriculture sector. They serve as a bridge between the rural and urban worlds, shedding light on the realities of farming and rural life. They also help break down stereotypes about agriculture and rural areas, providing a more realistic and nuanced perspective.
2.1 Educating the Public
One of the primary roles of agroinfluencers is to educate the public about agriculture and rural life. They share their knowledge about various agricultural practices, including crop cultivation, livestock rearing, and agroforestry. They also discuss the challenges and opportunities in agriculture, providing valuable insights for both farmers and non-farmers alike.
2.2 Advocacy for Sustainable Practices
Many agroinfluencers are strong advocates for sustainable agricultural practices. They promote methods that enhance agricultural productivity while minimizing negative environmental impacts. They also highlight the importance of biodiversity conservation, soil health, and water conservation in agriculture.
2.3 Marketing and Promotion
Agroinfluencers often collaborate with agricultural brands and companies for marketing and promotion. They might use their platform to showcase a particular agricultural product, demonstrate its usage, or share their personal experiences with it. They can effectively increase the visibility of these brands and products, reaching a wider audience than traditional advertising methods.
3. The Impact of AgroInfluencers
Agroinfluencers have a significant impact on the agriculture sector and beyond. They can shape public opinion, influence consumer behavior, and even affect policy decisions.
3.1 Shaping Public Opinion
Agroinfluencers can significantly shape public opinion about agriculture and rural life. By sharing their experiences and insights, they can challenge common stereotypes, dispel misconceptions, and provide a more accurate portrayal of farming and rural life. This can lead to increased appreciation and support for farmers and the agricultural sector.
3.2 Influencing Consumer Behavior
Agroinfluencers can also influence consumer behavior. For example, they can promote local and sustainably produced agricultural products, influencing consumers to make more environmentally-friendly purchasing decisions. They can also raise awareness about issues such as food waste and food security, prompting consumers to adopt more responsible consumption habits.
4. Some of our female AgroInfluencers
There are numerous agroinfluencers making significant contributions to the agriculture sector. Here are some noteworthy examples:
4.1 Cristina Stribacu
 Cristina Stribakou is the founder of the LIÁ Premium Olive Oil company, known for its award-winning extra virgin olive oil produced in the olive groves owned by LIÁ Cultivators in Messinia, Greece. Cristina studied Art History and Italian, and when her entrepreneurial spirit faced the challenge of implementing sustainable and environmentally friendly practices on her farm, she was fully committed. She is a passionate, dedicated and forward-looking professional whose aim is to provide high-quality services and offer LIÁ premium extra virgin olive oil, a world-class product, with respect for authenticity and nature.
Cristina Stribakou is the founder of the LIÁ Premium Olive Oil company, known for its award-winning extra virgin olive oil produced in the olive groves owned by LIÁ Cultivators in Messinia, Greece. Cristina studied Art History and Italian, and when her entrepreneurial spirit faced the challenge of implementing sustainable and environmentally friendly practices on her farm, she was fully committed. She is a passionate, dedicated and forward-looking professional whose aim is to provide high-quality services and offer LIÁ premium extra virgin olive oil, a world-class product, with respect for authenticity and nature.
https://www.instagram.com/cristina_stribacu
https://www.linkedin.com/in/cristinastribacu/?originalSubdomain=gr
4.2 Ayala Noy Meir
 Ayala Noy Meir is a leading agronomist with a master's degree from the Hebrew University of Jerusalem. She has specialised in the field of olive oils, being a professional taster and leading the panel of the International Olive Oil Council.
Ayala Noy Meir is a leading agronomist with a master's degree from the Hebrew University of Jerusalem. She has specialised in the field of olive oils, being a professional taster and leading the panel of the International Olive Oil Council.
In addition to her academic role, Noy Meir is the Manager of the "Rish Lakish" organic olive oil mill. In this role, she oversees the cultivation of over 100 acres of organic olive trees, with a diversity of 8 varieties.
His experience and expertise has also extended to teaching, being a lecturer on olive oil at various forums. Ayala Noy Meir also brings her experience as a quality consultant to olive oil producers in Israel.
Her reputation and skills have made her a highly regarded judge in national and international olive oil competitions. In short, Ayala Noy Meir is a professional with a deep passion for excellence in olive oil, dedicated to quality and to the promotion of organic and sustainable practices.
https://www.instagram.com/noymeirayala
https://www.linkedin.com/in/ayala-noy-meir-
4.3 Emilie Borel Berta
 Emilie Borel-Berta is passionate about Mediterranean culture and olive growing. Originally from Corsica, Emilie has transformed 35 hectares of land into a thriving olive oil production business. With the help of Italian expert Ivo Berta, Emilie built a mill using state-of-the-art technology to produce olive oil of the highest quality. Through her hard work and dedication, Emilie has managed to cultivate olive trees on land that was once devoid of them. Today, her organic mill attracts visitors from all over the world and her olive oil is available through various delivery options. His love for the history and culture of Corsica is reflected in every bottle of his precious olive oil.
Emilie Borel-Berta is passionate about Mediterranean culture and olive growing. Originally from Corsica, Emilie has transformed 35 hectares of land into a thriving olive oil production business. With the help of Italian expert Ivo Berta, Emilie built a mill using state-of-the-art technology to produce olive oil of the highest quality. Through her hard work and dedication, Emilie has managed to cultivate olive trees on land that was once devoid of them. Today, her organic mill attracts visitors from all over the world and her olive oil is available through various delivery options. His love for the history and culture of Corsica is reflected in every bottle of his precious olive oil.
https://www.instagram.com/emilieborelberta
https://www.linkedin.com/in/emilie-borel-531a2815b/
4.4 María Lanagran

Maria Lanagran, daughter of ARTOLIO's renowned Extra Virgin Olive Oil (EVOO) producer Blas Lanagran, is a passionate and dedicated professional. As the new generation of the family production, Maria is determined to push the family business forward and give a more prominent role to women in the sector. Her academic background in chemistry and specialisation in elaiotechnology, combined with her role as a researcher in the area of food technology in the Department of Chemical, Environmental and Materials Engineering at the University of Jaén, positions her as a key figure in the advancement of the olive oil sector. Her dedication to the study of the quality and traceability of olive oil, and her focus on creating innovative strategies to improve its production, highlight her commitment to excellence and innovation. Maria Lanagran is undoubtedly an emerging leader in the olive oil industry.
https://www.linkedin.com/in/maria-lanagran-perea-8b7621180/
5. The Future of AgroInfluencers
The role of agroinfluencers in the agriculture sector is likely to grow in the future. As social media continues to evolve and expand, more individuals will have the opportunity to share their experiences and insights about agriculture and rural life.
Agroinfluencers will continue to play a crucial role in educating the public, advocating for sustainable practices, and promoting agricultural products. They will also continue to shape public opinion and influence consumer behavior in ways that benefit the agricultural sector.
Moreover, as the digital divide in rural areas continues to narrow, more farmers and agricultural professionals will have access to social media and the opportunity to become agroinfluencers. This will further diversify the voices and perspectives in the agricultural discourse, fostering a more inclusive and comprehensive understanding of agriculture and rural life.
The rise of agroinfluencers signifies a new era in agricultural communication. It reflects the growing recognition of the importance of agriculture and rural life in our society. It also highlights the power of social media in bridging the urban-rural divide and bringing agriculture closer to the public.
In conclusion, agroinfluencers are more than just social media personalities. They are advocates, educators, and change-makers in the agricultural sector. They are the emerging voices in the agricultural landscape, shaping public opinion, influencing consumer behavior, and driving positive change in agriculture and rural life.
Content created by:
Jesus F. Gordillo
Communication Manager of ARTOLIO ENI CBC MED
Strategic Director of Kellenföl Ad, an agency based in Barcelona. Advisor to companies and public institutions. Professor of marketing and advertising strategies.
I am a producer, can I join the ARTOLIO platform?
Welcome producers to the ARTOLIO platform: Registration as from 1 September 2023
Dear oil producers, we are pleased to announce that from 1 September 2023, you will be able to apply to join the innovative ARTOLIO platform. This platform is designed to address the needs of all oil producers, especially small producers looking to expand their reach and improve their productivity.
Who can apply?
The ARTOLIO platform is open to all small oil producers who wish to expand their horizons and become part of our growing community. No matter how small your production is, if you produce quality oil, we want you to be part of our platform.
How to apply?
To apply for registration on the ARTOLIO platform, a simple and easy to fill out form will be made available. This form will allow us to review each case and ensure that all our members comply with our high quality standards and sustainable production practices.
Benefits of joining ARTOLIO ENI CBC MED
Once you join our platform, you will become a member of the prestigious ARTOLIO ENI CBC MED community. This not only gives you significant recognition in the oil industry, but also access to a number of benefits.
As a member of ARTOLIO ENI CBC MED, they will have access to resources and opportunities to improve their production efficiency. You will also have the opportunity to connect with other producers, share experiences, learn from experts and participate in exclusive community events.
Join us
If you are an oil producer looking to grow and thrive in the competitive oil market, there is no better place to do it than at ARTOLIO.
We look forward to receiving your application from 1 September 2023. Together, we can do great things and take the art of oil production to new heights.
10 ideas on how to give visibility to your olive oil
10 ideas on how to give visibility to your olive oil
Diving into the world of olive oil can be an exciting and rewarding adventure, but with so many brands and varieties on the market, it can be difficult to differentiate the product and make it stand out. We have compiled a list of 10 basic ideas to increase the visibility of your olive oil. Let's embark on this tasty journey and discover the secrets to unlocking the full potential of your product and making it the preferred choice of both enthusiasts and casual consumers.

- Story and quality: Share the story behind your olive oil, highlighting the tradition and quality of the ingredients used. This helps to establish an emotional connection with customers and highlight the authenticity of the product.
- Live demonstrations: Organize live demonstrations, such as oil tastings or cooking workshops, to show customers how to use your olive oil in different recipes and the advantages of its flavor.
- Collaborations with local chefs: Collaborate with local chefs to create exclusive recipes with your product. This can increase the visibility of the product and demonstrate its versatility in the kitchen.
- Social networks: Use social networks to promote your olive oil, sharing recipes, pictures and testimonials from satisfied customers. This can increase exposure and make your product more interesting to the public.
- Participation in fairs and events: Attend local fairs and events related to agriculture and food, where you can present your oil and publicize its benefits.
- Attractive labeling and packaging: Design eye-catching labels and packaging that stand out on store shelves and attract customers.
- Promotions and discounts: Offer special promotions and discounts on your oil, such as introductory offers, volume discounts or coupons.
- Partnerships with stores and restaurants: Establish partnerships with local stores and restaurants to sell and use your olive oil in their recipes. This can increase product demand and visibility.
- Social and environmental responsibility: Emphasize sustainable and ethical production practices, such as using local ingredients, recycling packaging or minimizing waste. This can attract customers concerned about the environment and social responsibility.
- Loyalty program: Implement a loyalty program to reward frequent customers with discounts, gifts or exclusive promotions. This will build customer loyalty and encourage repeat purchases.
Content created by:
Jesus F. Gordillo
Communication Manager of ARTOLIO ENI CBC MED
Strategic Director of Kellenföl Ad, an agency based in Barcelona. Advisor to companies and public institutions. Professor of marketing and advertising strategies.
A High Temperature Environment Regulates the Olive Oil Biosynthesis Network
A High Temperature Environment Regulates the Olive Oil Biosynthesis Network
Yael Nissim 1, Maya Shlosberg 1,2, Iris Biton 1, Yair Many 1, Adi Doron-Faigenboim 1, Ran Hovav 1, Zohar Kerem 2, Benjamin Avidan 1 and Giora Ben-Ari 1,*
- 1 Institute of Plant Sciences, ARO, The Volcani Center, Rishon LeZion 7528809, Israel; yaelns@volcani.agri.gov.il (Y.N.); Maya.shlosberg@mail.huji.ac.il (M.S.); ivrb28@volcani.agri.gov.il (I.B.); yairm@volcani.agri.gov.il (Y.M.); adif@volcani.agri.gov.il (A.D.-F.); ranh@volcani.agri.gov.il (R.H.); vhavidan@volcani.agri.gov.il (B.A.)
- 2 Institute of Biochemistry, Food Science and Nutrition, The Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rehovot 76100, Israel; zohar.kerem@mail.huji.ac.il
* Correspondence: giora@agri.gov.il
Abstract: Climate change has been shown to have a substantial impact on agriculture and high temperatures and heat stress are known to have many negative effects on the vegetative and reproductive phases of plants. In a previous study, we addressed the effects of high temperature environments on olive oil yield and quality, by comparing the fruit development and oil accumulation and quality of five olive cultivars placed in high temperature and moderate temperature environments. The aim of the current study was to explore the molecular mechanism resulting in the negative effect of a high temperature environment on oil quantity and quality. We analyzed the transcriptome of two extreme cultivars, ‘Barnea’, which is tolerant to high temperatures in regard to quantity of oil production, but sensitive regarding its quality, and ‘Souri’, which is heat sensitive regarding quantity of oil produced, but relatively tolerant regarding its quality. Transcriptome analyses have been carried out at three different time points during fruit development, focusing on the genes involved in the oil biosynthesis pathway. We found that heat- shock protein expression was induced by the high temperature environment, but the degree of induction was cultivar dependent. The ‘Barnea’ cultivar, whose oil production showed greater tolerance to high temperatures, exhibited a larger degree of induction than the heat sensitive ‘Souri’. On the other hand, many genes involved in olive oil biosynthesis were found to be repressed as a response to high temperatures. OePDCT as well as OeFAD2 genes showed cultivar dependent expression patterns according to their heat tolerance characteristics. The transcription factors OeDof4.3, OeWRI1.1, OeDof4.4 and OeWRI1.2 were identified as key factors in regulating the oil biosynthesis pathway in response to heat stress, based on their co-expression characteristics with other genes involved in this pathway. Our results may contribute to identifying or developing a more heat tolerant cultivar, which will be able to produce high yield and quality oil in a future characterized by global warming.

1. Introduction
Abiotic stresses, including high temperatures have a substantial negative impact on the reproductive phase of the plant. Oilseed crops are also negatively affected by heat stress, which has been shown to reduce starch, protein and oil content [1,2]. Plants exposed to temperatures above their optimal growing temperatures, exhibit cellular and metabolic responses which enable the plants to survive [3–7]. The level of damage to crops caused by high temperatures depends on many parameters. The reproductive phase of plant development is more sensitive to high temperatures than the vegetative phase, causing a reduction in yield [8]. Yield reduction in response to heat stress or high temperatures has been reported in wheat, peanut, rice bean and tomato [9–13]. Olive oil accumulation in the mesocarp cells of the fruit is influenced by cultivar type and climactic conditions. It occurs mainly during the summer and decreases during fruit ripening in the fall [14,15].
Olive oil quality is characterized by its sensorial and nutritional properties. Its health benefits are due to both its fatty acid composition, especially oleic acid concentration and minor compounds such as polyphenols. These are strongly affected by factors such as cultivar type, degree of ripeness, fruit load, and soil quality [16]. Several studies have examined the effect of high temperatures on olive oil accumulation [17–20] and olive oil fatty acid composition [17,19,21–24]. Oleic acid concentration in olive oil was found to be negatively affected by a high temperature environment [17].
1.1. Heat-Shock Proteins
Plants interact with climatic factors such as heat stress by triggering certain mechanisms of defense such as a specific gene expression program. A response to heat stress on the molecular level is found in all living organisms and results in an increase in the induction and synthesis of a group of proteins called heat-shock proteins [25]. Heat-shock proteins positively regulate antioxidant enzymes which detoxify reactive oxygen species. They also enhance plant immunity by the accumulation and stability of pathogenesis-related proteins produced under biotic stresses [26]. During the onset of stress, plants reduce normal protein production, and transcribe and translate heat-shock proteins. Heat-shock proteins have been reported in a wide range of organisms and are highly conserved [26]. Based on molecular weight, HSPs are generally classified into the following sub-families: HSP100, HSP90, HSP70, HSP60, and small HSPs. Several studies have indicated that many high molecular weight HSPs exhibited a response to high-temperature stress [26]. Among the many heat-shock proteins, HSP70s and HSP60s whose response to heat stress has been most intensively studied, have been shown to maintain proper folding under conditions of heat stress with the aid of ATPs [26]. Hsp70s are a class of highly conserved proteins which act as molecular chaperones and play a crucial role in protecting the plant cells from the harmful effects of heat stress. Hsp70 is known to accumulate in heat-stressed tissue and overexpression of Hsp70 was shown to enhance tolerance to heat stress in several plant species including brassica, tobacco and rice [27–30].
1.2. Olive Oil Biosynthesis
Fatty acid biosynthesis begins with Acetyl-CoA carboxylase (ACCase) catalyzing the formation of malonyl-CoA from acetyl-CoA by the ACCase enzyme, which is a complex consisting of three separate proteins or domains: BC, CT and BCCP. ACCase has two isoforms, the prokaryotic enzyme is a heteromeric multisubunit complex (htACC). In eukaryotic cells, the enzyme takes the form of a homomeric multidomain polypeptide (hmACC). The hmACC is a single multifunctional polypeptide, with all three domains forming part of a single polypeptide (ACC1). htACC consists of four separate proteins, BC, BCCP, and CT, which is a heterodimer with α and β subunits. The majority of plants require both types of ACCase, localizing htACC in plastids and hmACC in the cytosol [31]. The MCAT enzyme catalyzes formation of malonyl-ACP from malonyl-CoA. Fatty acids are subsequently produced by a complex of six enzymes referred to as fatty acid synthase (FAS). This complex includes KAS I, KASII and KASIII, KAR, HAD and ENR enzymes. The first step is the condensation of malonyl-ACP with acetyl- CoA by the action of KAS III to produce acetoacetyl-ACP in the plastid. This subsequently forms a β-hydroxyacyl derivative by KAR, which is then dehydrated by HAD and finally reduced by ENR, resulting in a four-carbon acyl-ACP derivative. This forms 3- Katoacyle-ACP by the action of KAS I which can undergo five more chain elongation cycles. The final product of these cycles is palmitoyl-ACP with 16 carbons (C16:0-ACP). Finally, this compound can be elongated to form stearoyl-ACP (C18:0) by KAS II. The most abundant fatty acid in olive oil, oleic acid, is formed by SAD enzyme. The release of the acyl segment from the ACP derivatives is performed by FATA and FATB. These thioesterases show different substrate specificities. FATA preferentially hydrolyzes the 18:1 acyl-ACP, whereas the FATB type is most active with 16:0 and 18:0 acyl-ACPs. The process is localized on the endoplasmic reticulum (ER) by a reaction catalyzed by an oleate desaturase (FAD2) to form linoleic acid (18:2) from oleic acid 18:1. Further desaturation may occur, catalyzed by FAD3 to produce α-linolenic acid (18:3). The assembly of triacylglycerol (TAG) from glycerol-3-phosphate and fatty acids take place on the ER, by the Kornberg–Pricer acylation reactions followed by the Kennedy pathway mediated by the enzymes GPDH, GPAT, LPAAT and PP to form diacylglycerol (DAG). Finally, DAG is an important precursor for synthesis of phospholipids such as PC or storage lipids such as TAG. In order to form TAG, DAG is acylated at the third position by a diacylglycerol acyltransferases (DGATs) or by a phospholipid; diacylglycerol acyltransferase (PDAT) [32]. Another pathway for the DAG is through reactions of phosphatidylcholine diacylglycerol cholinephosphotransferase (PDCT), in which the acyl groups enter PC and can then return to DAG after they are desaturated or otherwise modified on PC [33].
1.3. Transcription Factors Regulate Olive Oil Biosynthesis
Regulation of oil biosynthesis by transcription factors (TFs) has been widely investigated and many transcription factors have been reported to affect TAG production [34]. Leafy cotyledon 1 (LEC1) and LEC1-LIKE (L1L) TFs have been reported to regulate fatty acid synthesis through ACCase in Arabidopsis, maize and bean. DOF4 and DOF11 TFs were found to regulate fatty acid synthesis through ACCase, ENR and KASII in Arabidopsis and soybean. MYB73 was reported to regulate fatty acid synthesis through FATB and KAS genes in Arabidopsis and Camelina sativa [34]. However, WRINKLED1 (WRI1) transcription factor is vital to synthesis, and is the “master regulator” of the transcriptional control of the plant oil biosynthesis pathway [35]. Several other TFs were reported to control fatty acid biosynthesis not directly through the biosynthesis genes, but through other genes or transcription factors. These TFs include LEC2, FUS3, ABI3, several bZIP TFs, MYB96, MYB30, PICKLE, VAL1, VAL2, ASIL1 and AP2 [34].
We have lately addressed the effect of high temperature environments on olive oil yield and quality, by comparing the fruit development and oil accumulation and quality of five olive cultivars placed in high temperature (HT) and low temperature (MT) environments [36]. We found that the effects of a high temperature environment are genotype dependent and in general, high temperatures during fruit development decreased oil accumulation and quality. ‘Barnea’ cultivar final oil content was not affected by the high temperature environment, but ‘Souri’ cultivar exhibited a dramatic decrease of 8% in oil concentration calculated as a percentage of dry fruit weight at harvest in response to high ambient temperatures. Regarding the quality of oil produced, the ‘Souri’ cultivar proved more tolerant to a high temperature environment than any other of the tested cultivars. The ‘Barnea’ oil had a polyphenol level of 404 mg/g oil in the MT environment and only 156 mg/g oil in the HT environment. In addition, the ‘Barnea’ oil showed a decrease of 4.8% in oleic acid concentration of oil extracted from fruit grown in HT conditions compared to the MT environment. The ‘Souri’ cultivar oil contained high polyphenol levels of 905 and 772 mg/g oil in the MT and HT environments respectively. In addition, the ‘Souri’ oil showed a decrease of only 3.7% in oleic acid concentration of oil extracted from fruit grown under HT conditions compared to oil from MT fruit. The aim of the current study was to explore the molecular mechanism underlying the negative effect of the high temperature environment on oil quantity and quality. In order to identify the molecular mechanism and the possible blocks, in which HT negatively affects oil accumulation and quality, we analyzed the transcriptome of the two extreme cultivars, ‘Barnea’ and ‘Souri’. Transcriptome analyses were carried out at three different time points during fruit development, focusing on the genes involved in the oil biosynthesis pathway.

2. Materials and Methods
2.1. Experimental Design and Plant Material
The olive cultivars ‘Barnea’, ‘Coratina’, ‘Koroneiki’, ‘Souri’ and ‘Picholine’ were used in this study. Of these, ‘Barnea’, which is tolerant to HT environment, regarding fruit development and oil accumulation and ‘Souri’, tolerant to HT environment, regarding oil quality, were used for transcriptome analysis. Twelve olive trees in 50 L pots of each of the five cultivars were placed in an extremely hot climate zone at Tirat Zvi village (HT environment) and in a more temperate environment (MT environment) during 2016 and 2017. Experiment design and all measurements were described previously in detail [36]. During 2017, the mesocarp of five fruits from each of the six trees was collected and three replicates of mesocarp from a mix of ten fruits from two trees each were used for transcriptome analysis. Mesocarp was sampled at three time points during the season, at 83, 104 and 146 days post anthesis (DPA).
2.2. Transcriptome Analysis
Fruits were removed from the tree, the mesocarp of a pool of 10 fruits from two trees was excised, mixed in a tube and immediately frozen in liquid nitrogen. Total RNA was extracted from olive mesocarp tissue using Sigma (Sigma Aldrich, Darmstadt, Germany) plant total RNA extraction kit according to the manufacturer’s instructions. DNA was removed by treatment with RNAase-free DNAase at 37 °C for 30 min. Total RNA samples were used to prepare 24 cDNA libraries of the two cultivars (‘Barnea’ and ‘Souri’) at three time points during fruit development (83, 104 and 146 DPA) in two locations (HT and MT environments) and two biological replicates, using Illumina’s TruSeq RNA library preparation kit according to the manufacturer’s instructions. cDNA libraries were sequenced on NextSeq high output 75 cycles. Sequencing was performed by the Sequencing unit of the Crown Institute for Genomics in the Weizmann Institute of Science, Israel. Raw reads were subjected to a filtering and cleaning procedure using the FASTX Toolkit (http://hannonlab.cshl.edu/fastx_toolkit/, version 0.0.13.2) for: (1) trimming read-end nucleotides with quality scores <30 using fastq_quality_trimmer; (2) removing reads with less than 70% base pairs with quality score ≤30 using fastq_quality_filter. Cleaned reads, obtained after processing, were assembled de novo using Trinity software (GitHub Inc., San Francisco, CA, USA) [37] with trimmomaticSE option to remove adaptors [38]. Only transcripts with a minimum length of 200 bp were analyzed against OE6 transcriptome reference. The transcript quantification (the number of reads per gene) from RNA-Seq data was performed using bowtie2 aligner [39] and the Expectation- Maximization method (RSEM) [40]
2.3. RT-PCR
Specific primers were designed using Primer3 software (Elixir, Narva maantee, Estonia) [41]. Primers for the various genes were designed to amplify a region containing more than one exon, so that genomic DNA would not be amplified. A total of 2.5 μg RNA from each sample was treated with RQ DNase (Promega, Madison, WI, USA) and reverse-transcribed using random hexamer primers (Promega). Real-time PCR was carried out using the SYBR green amplification kit (ABgene, Blenheim Road, Epsom, UK) according to manufacturer’s instructions. Each reaction contained 1 μL cDNA and 1 μM of each primer from the relevant primer pair in a final volume of 10 μL. Quantification of real- time PCR products was carried out by detection of SYBR green fluorescence on a StepOnePlusTM system (Applied Biosystems, Foster City, CA, USA). Dilution series of cDNA were created for each set of and a standard curve was established for each gene. Triplicate of cDNA were used and each reaction was subjected to melting-point analysis to confirm single amplified products. At least two biological repeats were carried out for each gene. Transcript levels were estimated using a standard curve for each gene, and these levels were normalized against the amount of OeACTIN transcript level in each sample. The sequences of the primers used are listed in the Table S1.
2.4. Data Analysis
Differential expression analysis was done with edgeR package (Bioconductor, Buffalo, NY, USA) [42], transcripts that were more than two fold differentially expressed with false discovery corrected statistical significance of at most 0.01 were considered differentially expressed [43]. Hierarchical clustering of gene expression and visualization of heat map were performed using R Bioconductor (Bioconductor, Buffalo, NY, USA) [44], using the normalized log-FPKM values (Z score) of each gene. Venn diagrams were constructed using the online Venny 2.0 software (http://bioinfogp.cnb.csic.es/tools/venny/). From each gene family participating in the oil biosynthesis pathway, we presented only genes that showed a normalized expression level of at least 200 FPKM in at least one time-point and environment, assuming that genes with low expression levels are not involved in the biosynthesis pathway. Co-expression network analysis inferred by using Conet plugin of cytoscape software (Cytoscape.js, Toronto, ON, Canada) that was based on a Pearson correlation (cut-off of r > 0.75).
2.5. Statistical Analysis
RT-PCR statistical analyses were conducted using JMP software (SAS, Cary, NC, USA) [45]. The RT-PCR results were subjected to two-way analysis of variance (ANOVA) including full factorial analysis, for their dependence on the two independent variables of tree location and cultivar type, including the interaction between them. Since we encountered significant interaction between the two factors in all gene expression analysis, a Tukey-Kramer test, based on multiple comparison correction was performed, in order to compare the various levels of interaction.
3. Results
3.1. Gene Expression Regulation
In order to understand the mechanism in which high temperature summers affect olive oil biosynthesis we used transcriptome analysis. Two out of five analyzed [36] olive cultivars were chosen, ‘Barnea’ and ‘Souri’. ‘Barnea’ exhibited tolerance to HT environment in term of oil concentration at harvest, but its oil quality dramatically decreased in an HT environment. ‘Souri’ exhibited sensitivity to HT environment in term of oil concentration at harvest, but proved more tolerant to HT in oil quality, compared to all the tested cultivars. Mesocarp transcriptomic of the two cultivars was analyzed at three time points during fruit development in HT and MT environments, 83, 104 and 146 days post anthesis (DPA).
The transcriptome sequencing resulted in an average of 21,748,879 reads for each sequenced sample. Among them, 96.54% were clean reads and 83.47% of the reads were significantly mapped to the olive genome [46] (Table S2). The sequencing data were deposited in the NCBI Sequence Read Archive (SRA) database as bioproject PRJNA638790.
Comparing expression levels of each gene in the HT and MT environments for each cultivar and each time point, identified the regulated genes in response to different environments. The ‘Barnea’ cultivar had 2437, 2795 and 3265 genes that were significantly upregulated in the HT environment at 83, 104 and 146 DPA respectively. The ‘Souri’ cultivar had 3009, 2625 and 3129 genes that were significantly upregulated in the HT environment at 83, 104 and 146 DPA respectively. The ‘Barnea’ cultivar had 2500, 2634 and 3473 genes that were significantly upregulated in the MT environment at 83, 104 and 146 DPA respectively. The ‘Souri’ cultivar had 2953, 3053 and 3174 genes that were significantly upregulated in the MT environment at 83, 104 and 146 DPA respectively. Among the differentially expressed genes, more genes were up-regulated at all three time points (1193, 1226, 1291 and 1362 for ‘Barnea’-MT-Up, ‘Barnea’-HT-Up, ‘Souri’-MT-Up and ‘Souri’-HT-Up respectively) compared to those that were unique for specific time point or common to only two time points (Figure S1a). Comparing the identity of the regulated genes between the three selected time points showed that the highest number of regulated genes is common at all three time points. However, when comparing the regulated genes between the two cultivars, we found that the number of regulated genes that were cultivar specific in each of the two cultivars was similar to the number of regulated genes common to both cultivars, in all three time points (Figure S1b).
3.2. Heat-Shock Protein Genes are Upregulated in the HT Environment
Hierarchical clustering of all 110 heat-shock protein genes revealed that the gene expression profile of the heat-shock protein genes in ‘Barnea’ and ‘Souri’ in all three time points in the MT environment is similar (Figure 1). Most of these genes were expressed at low rates in both cultivars and at all three time points. In addition, The MT samples were separated by cultivar into two cluster groups. For the ‘Souri’, three time points in MT were clustered in one group whereas for ‘Barnea’, three time points in MT were clustered in another group. In the HT samples, ‘Souri’ at 104 DPA and ‘Barnea’ at 83 DPA clustered together and most of the heat-shock protein genes were expressed at low rates in these samples. ‘Souri’ at 83 and 146 DPA and ‘Barnea’ at 104 DPA were clustered in one group and most of the heat-shock protein genes were highly expressed. Above all, the heat-shock protein genes of ‘Barnea’ at 146 DPA in the HT environment were expressed at extremely high rates. Validation of the heat-shock protein gene expression pattern in HT and MT environments was carried out by RT-PCR analysis of the expression pattern of OeHSP70 known to be induced by heat shock.
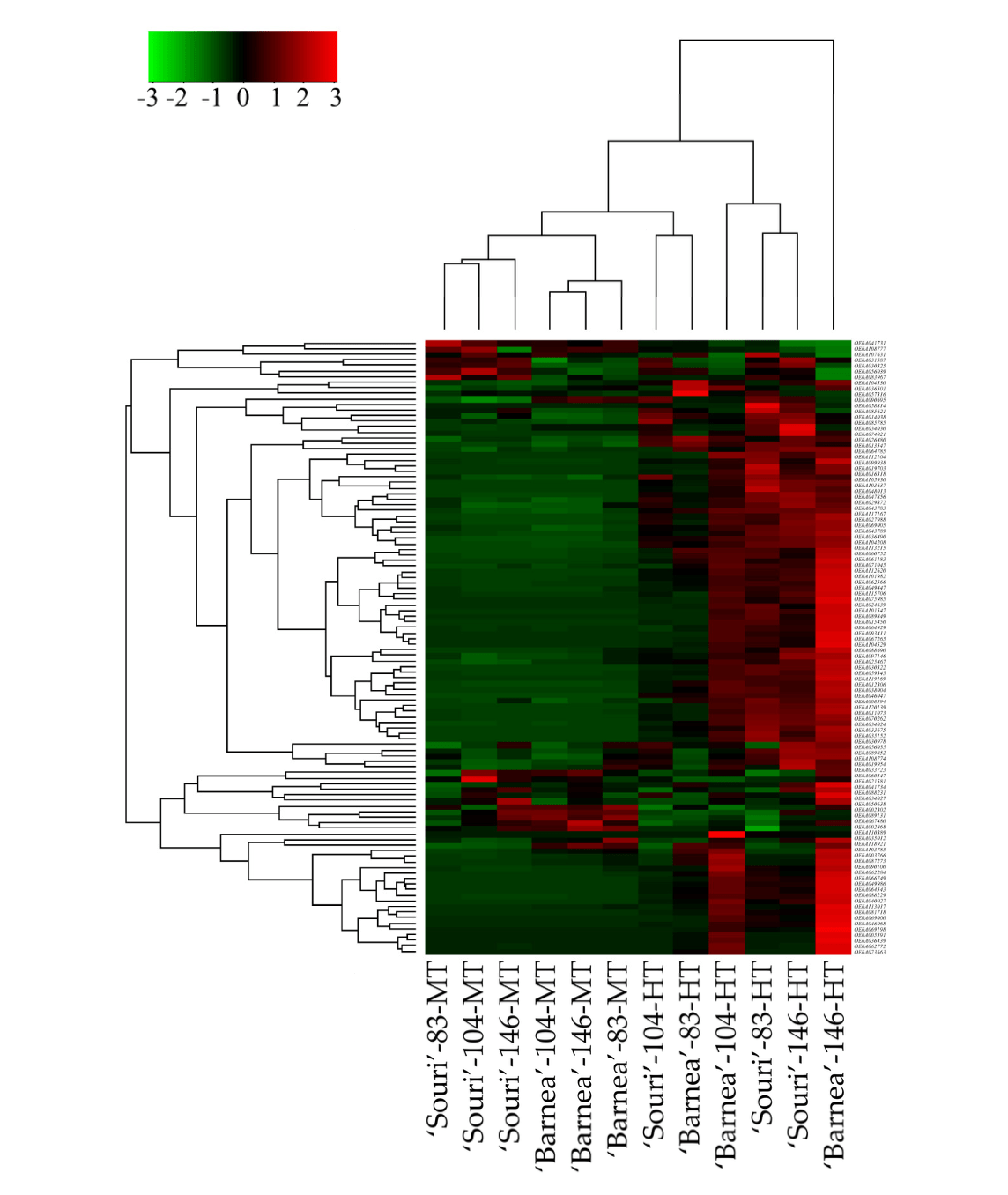
Expression level of OeHSP70 (OE6A062772) at 146 DPA, in all five cultivars [36] was analyzed (Figure S2). OeHSP70 expression level in the MT environment in all five cultivars was similar and relatively low compared to its expression in the HT environment. The expression level of OeHSP70 in the HT environment varied between the cultivars. The ‘Souri’ cultivar showed the lowest expression, ‘Picholine’ and ‘Coratina’ exhibited moderate expression, and ‘Barnea’ and ‘Koroneiki’ showed the highest expression, in which, ‘Barnea’ OeHSP70 expression was found to be 8.7 times greater than OeHSP70 expression in the ‘Souri’ cultivar in the HT environment.
3.3. The Effect of High Summer Temperatures on the Olive Oil Biosynthesis Pathway
The biochemical pathways leading to oil (triacylglycerol) synthesis in the olive fruit mesocarp involve many subcellular organelles and multiple enzymes, beginning with the precursor, acetyl- CoA and concluding with triacylglycerol (TAG). Based on the transcriptome analysis of ‘Barnea’ and ‘Souri’ at the two contrasting environments at three time points during fruit development, we focused on the gene expression pattern of all genes involved in olive oil biosynthesis (Figure 2). Figure 2 presents the expression pattern of the ‘Souri’ and the ‘Barnea’ cultivars. The following stages describe in detail oil synthesis in the ‘Souri’, a cultivar we have shown to be sensitive to a high temperature environment as reflected by oil biosynthesis [36]. The first step of the fatty acids biosynthesis is catalyzing the formation of malonyl- CoA from acetyl-CoA by Acetyl-CoA carboxylase (ACCase). Gene expression pattern of the genes involved in the multienzyme complex was similar in the HT and MT environments beside OeBCCP1 (OE6A092496), which was induced in the HT environment. On the other hand, OeACC1 (OE6A049983) was expressed three to six fold (depending on the sample date) higher in the MT environment compared to the HT. OeMCAT, which catalyzed malonyl-CoA was expressed similarly in both environments. Fatty acid synthase (FAS) is an easily dissociated multisubunit complex consisting of the monofunctional enzymes KASI, KASII, KASIII, KAR, HAD and ENR. OeHAD, OeENR and OeKAR were expressed similarly in the HT and MT environments at the first sample date (83 DPA), at which OeKAR expression was higher in the MT compared to the HT environment. However, OeKASI, OeKASII and OeKASIII expression was higher in the MT compared to the HT environment in all three sample dates. Both stearoyl-ACP Δ9- desaturase (SAD) family genes (OE6A020845 and OE6A118450) were very highly expressed in both environments. The last stage of de novo fatty acid synthesis is regulated by the acyl-ACP thioesterases, FATA and FATB. The two FAT genes, OeFATA and OeFATB were induced in the MT environment in comparison to their expression in the HT. This is especially true for the OeFATB gene (OE6A029754), which is expressed at a threefold level in the MT environment compared to its expression in the HT at all three time points. The modification of oleic acid (18:1) into linoleic acid (18:2) and linolenic acid (18:3) is catalyzed by the fatty acid desaturases FAD2 and FAD3 respectively in the Endoplasmic Reticulum (ER). OeFAD3 genes were not expressed in our samples. However, OeFAD2 genes showed higher expression in the HT compared to the MT environment, especially regarding OeFAD2-5 (OE6A098403) at the last sample date (146 DPA) and OeFAD2-1 (OE6A069627) at the first sample date (83 DPA). The expression level of OeFAD2-5 in the ‘Souri’ cultivar at 146 DPA was found to be 11,700 FPKM in the HT environment, significantly higher than the 1976 FPKM level found in the MT environment. Assembly of fatty acids onto the glycerol backbone to create triacylglycerol (TAG) begins with a series of reactions with dihydroxyacetone phosphate (DHAP) as a precursor and diacylglycerol (DAG) as the product with four enzymes involved (GPDH, GPAT, LPAAT and PP). The expression level of the main genes involved in this reaction, OeGPDH (OE6A105859) and OeGPAT (OE6A109037) was significantly higher in the MT environment compared to that in the HT. However, OeLPAAT gene family consists of one gene (OE6A051077) that was induced in the HT environment and another single gene (OE6A111035) significantly induced in the MT environment. OePP genes were marginally expressed in both environments at all three time points.
DAG induction can proceed along three different pathways, two of them with TAG as the final product, and one, regulated by PDCT, to return to the phosphatidylcholine pool. DAG can create TAG by the reaction of PDAT or by the reaction of the two DAG proteins, DGAT1 and DGAT2.
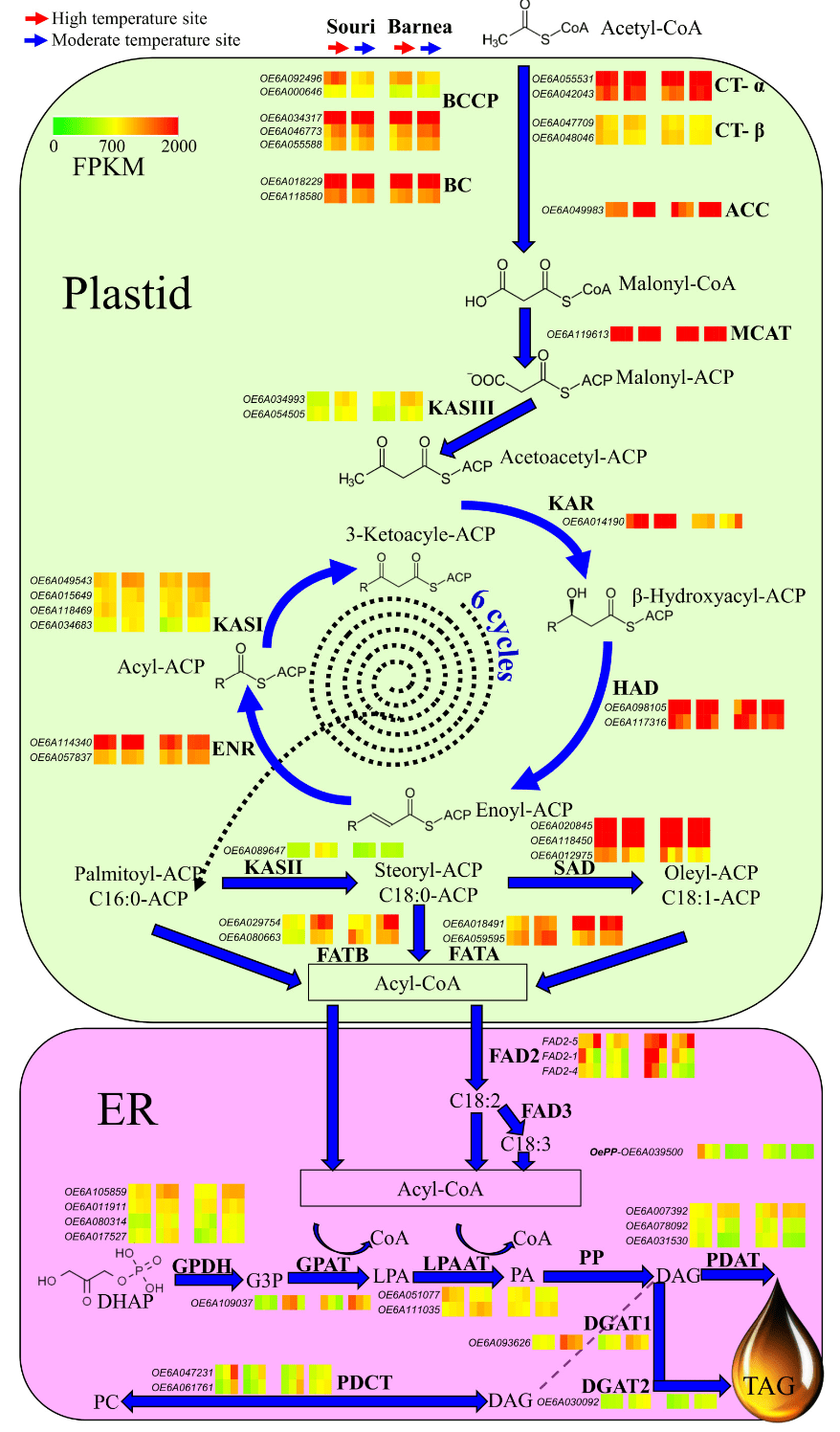
The OePDAT gene family consists of three genes that were highly expressed in our study. OE6A007392 had a significantly higher expression level in the MT compared to the HT environment at all three time points, whereas OE6A078092 and OE6A031530 had a significantly higher expression level in the HT compare to the MT. OeDGAT1 (OE6A093626) was significantly expressed at a higher level in the MT compare to the HT environment, especially at 83 DPA, whereas OeDGAT2 (OE6A030092) expression was higher in the MT only at the latter two time points at 104 and 146 DPA. The two expressed OePDCT genes (OE6A047231 and OE6A061761) showed higher expression levels in the HT compare to the MT environment at the time points 104 and 146 DPA.
The ‘Barnea’ gene expression pattern was similar to that of the ‘Souri’ with the same trends between the two environments (Figure 2). The exceptional genes were FATA, in which one, OeFATA (OE6A018491), was highly expressed in both environments at all three time points. The expression pattern of two FAD2 genes, OeFAD2-5 and Oe-FAD2-1 was also different in the ‘Barnea’ cultivar compared to the ‘Souri’. The ‘Barnea’ cultivar showed extremely high expression of OeFAD2-5 in the HT environment at all three time points and relatively high expression in the MT environment, especially at 146 DPA. Oe-FAD2-1 was very highly expressed in the first two sample dates (83 and 104 DPA) in the HT environment and moderately expressed in the MT environment at all time-points. The ‘Barnea’ cultivar showed the same trend in the expression of OeDGAT1 and OeDGAT2 in both environments as the ‘Souri’ cultivar, only in the ‘Barnea’ cultivar, the differences between the expression levels in the MT and HT environments were smaller. Unlike the ‘Souri’ cultivar, the ‘Barnea’ OePDCT genes were expressed similarly in both environments.
Validation of the oil biosynthesis gene expression pattern in HT and MT environment was carried out by RT-PCR analysis of the expression pattern of OeACC1 and OeFAD2-1 at 146 DPA, in all five cultivars [36] (Figure 3).
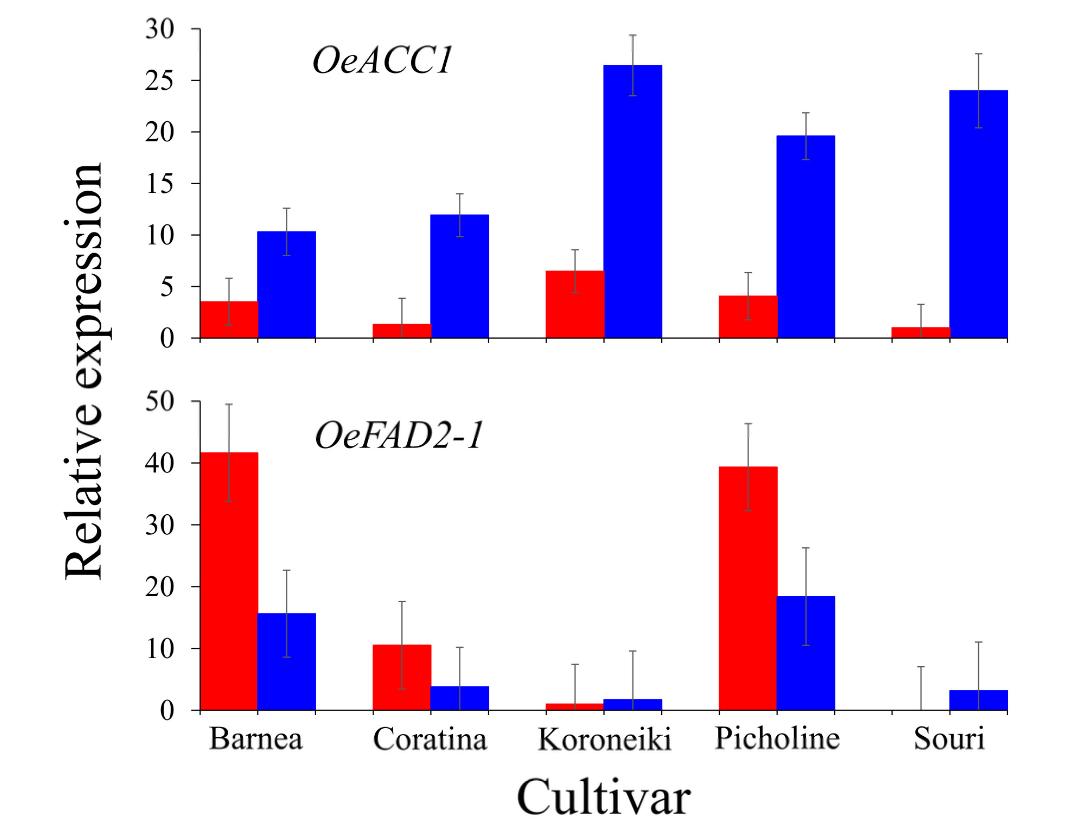
OeACC1 expression was significantly higher in the MT compared to the HT environment in all five cultivars, with the greatest differences between the two environments in the s ‘Koroneiki’ and ‘Souri’ cultivars. OeFAD2-1 expression level was relatively high in the MT environment in ‘Barnea’ and ‘Picholine’ cultivars and low in the other three. In the HT environment, OeFAD2-1 expression level in the ‘Koroneiki’ cultivar was low, and in the ‘Souri’ cultivar it was no expressed at all. In ‘Coratina’, it was expressed at a relatively high level and in the ‘Barnea’ and ‘Picholine’ cultivars was induced at extremely high levels.
3.4. Transcription Factors Regulating Olive Oil Biosynthesis Pathway
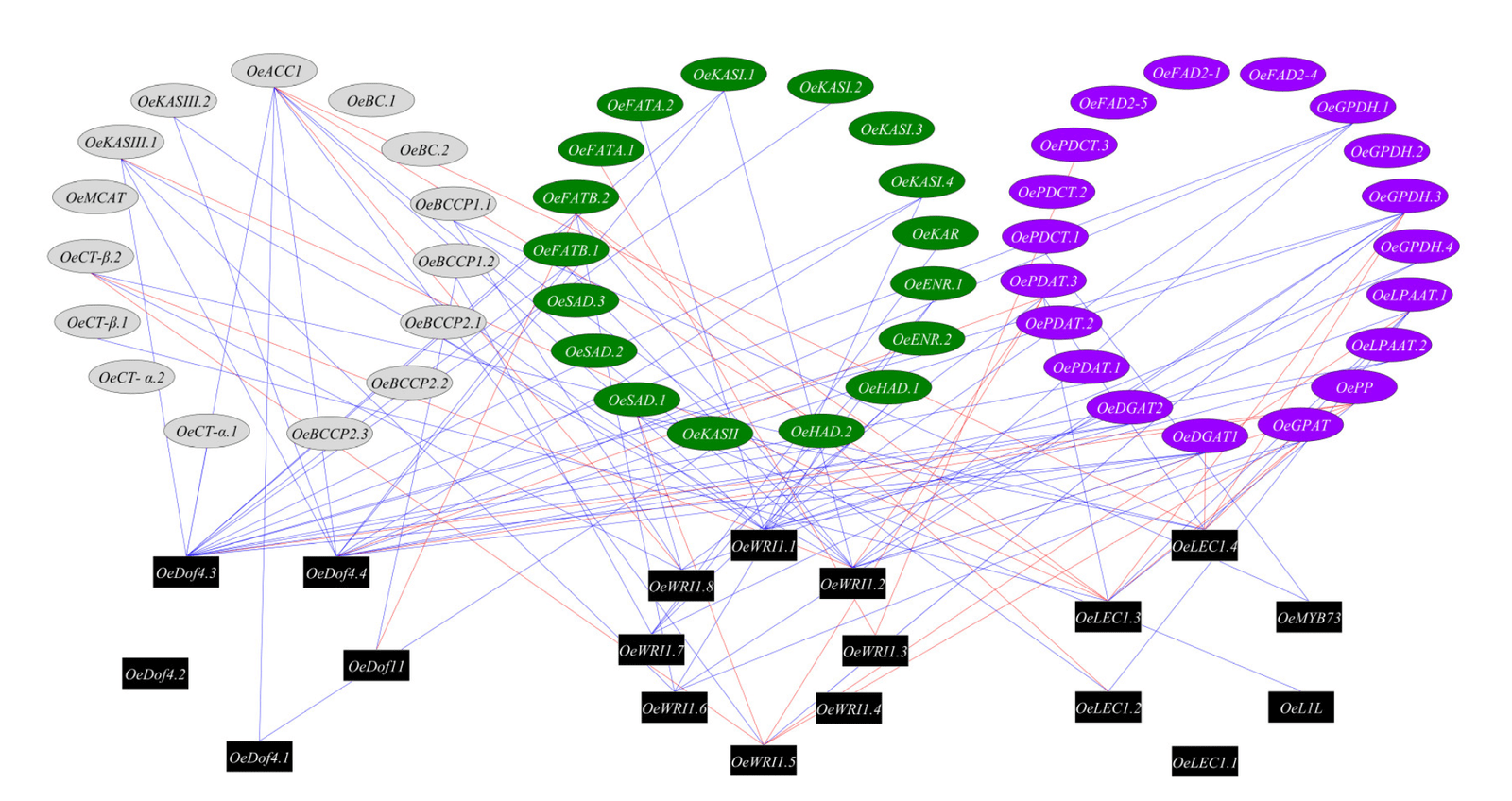
We next explored the regulatory roles of transcription factors in the oil biosynthesis pathway. In order to identify putative targets of the transcription factors known to directly regulate the oil biosynthesis pathway genes, we used co-expression analysis to identify significant and high (r > 0.75) expression pattern correlation between pairs of transcription factors and genes involved in the oil biosynthesis pathway (Figure 4). We identified eight WRI1 genes in the olive genome (OE6A039756, OE6A079258, OE6A099997, OE6A092113, OE6A061030, OE6A094680, OE6A054009 and OE6A025576), four Dof4 genes (OE6A082367, OE6A085395, OE6A104771 and OE6A039076), four LEC1 genes (OE6A094722, OE6A016910, OE6A001317 and OE6A033717), one Dof11 gene (OE6A032821), one L1L gene (OE6A077959) and one MYB73 gene (OE6A056119). The transcription factors with the highest number of putative target genes, based on co-expression, were OeDof4.3, OeWRI1.1, OeDof4.4 and OeWRI1.2 with 15, 12, 11 and 10 positive co-expressed oil biosynthesis genes. The oil biosynthesis genes with the highest number of putative transcription factors control, based on co-expression, were OeACC1, OeGPDH.3, OsDGAT1, OeFATB.2 and OePDAT.3 with 9, 7, 6, 6 and 6 co-expressed transcription factors.
4. Discussion
In this study, we addressed the molecular effects of an environment of consistently high temperatures on oil accumulation and composition in different cultivars. We used two cultivars that differ in their response to HT environment. The ‘Barnea’ cultivar, which had been found to be relatively resistant to HT environment in term of oil accumulation, but not in its oil quality and the ‘Souri’ cultivar, found to be sensitive to HT environment in term of oil accumulation, but relatively resistant to HT environment in term of oil quality [36]. We found that during fruit development and during the most critical period of oil accumulation, both cultivars, the heat tolerant ‘Barnea’ cultivar as well as the heat sensitive, ‘Souri’, induced a heat stress protein response in the HT environment. We characterized the gene expression pattern of the genes involved in the olive oil biosynthesis and found that many genes are repressed in response to a high temperature environment. Finally, we characterized the TF-oil biosynthesis network by its response to an MT vs. HT environment in the two analyzed cultivars, ‘Barnea’ and ‘Souri’.
4.1. HT Environment Induced A Heat Stress Response in Both Cultivars
Heat stress or high temperature affects the metabolism of plants, especially cell membranes and physiological processes such as photosynthesis, respiration, and water regulation. This defense at the molecular level is very important for survival and growth of plants. Plants show a series of molecular responses to these stresses, among them is the synthesis of heat-shock proteins. Heat-shock proteins act as molecular chaperones regulating the folding, accumulation, localization and degradation, of proteins in plants [25]. Our study focused on heat stress only. We treated the plant with enough water and fertilization to attempt to isolate heat stress from other abiotic stresses [36]. Characterization of the expression pattern of heat-shock proteins in our study, revealed that most of the heat-shock proteins induced dramatically in an HT environment were HSP20-like chaperones, although some genes belonging to the HSP70 family had the same expression pattern. Most heat-shock proteins in our study underwent high expression in the HT environment and low expression in the MT
environment at all three time-points (Figure 1). The ‘Souri’ cultivar presents a unique expression pattern of many HSP genes that were induced in 83 DPA, then repressed in 104 DPA, and up- regulated again in 146 DPA. We could not find any reasonable explanation for this gene expression pattern. At 104 and 146 DPA, the ratio between the expression of the heat-shock protein genes in the HT and the MT environment was higher in the ‘Barnea’ compared to the ‘Souri’ cultivar. Assuming that the ‘Barnea’ cultivar is more tolerant to heat stress compared to the ‘Souri’ cultivar in regard to fruit development and oil accumulation [36], suggests that induction of heat-shock proteins may contribute to the olive plant’s tolerance to heat stress. This assumption is in agreement with other studies which found that heat-shock proteins are involved in heat tolerance [26,47,48]. Analysis of OeHSP70 expression also showed the same pattern, in which the differences between the expression levels in the HT and the MT environments were much higher in the ‘Barnea’ cultivar than in the ‘Souri’. However, contrary to that, the ‘Koroneiki’ cultivar, which is as sensitive to high temperatures as the ‘Souri’ [36], showed a similar pattern of OeHSP70 expression as the ‘Barnea’ and not, as we might have predicted, to the ‘Souri’ (Figure S2). The OeHSP70 expression pattern in the ‘Souri’ and ‘Barnea’ cultivars as revealed in the RT-PCR experiment showed the same trend as was seen in the RNA-seq results. However, the ratio between the expression level in HT and MT environments was slightly different. This ratio was 1308:1 and 12:1 in the ‘Barnea’ and ‘Souri’ cultivars respectively according to the RNA-seq results and 630:1 and 101:1 respectively according to the RT-PCR results.
4.2. High Summer Temperatures Repress Genes Involved in the Olive Oil Biosynthesis Pathway
Formation of malonyl-CoA from acetyl-CoA is catalyze by ACCase. In our study, both the genes composing the multienzyme complex (htACC) as well as hmACC (OeACC1) were expressed in the olive mesocarp (Figures 2 and 3). It has been suggested that in monocotyledons both forms are active, whereas in dicotyledons, such as olives, hmACC alone is active [32]. However, according to the expression levels of both forms, our results suggest that in olives both forms may be active and contribute to the malonyl-CoA, a precursor of fatty acids. Many genes involved in olive oil biosynthesis are not sensitive to warm temperatures during fruit development and show similar expression in both HT and MT environments. These genes include the genes responsible for forming the htACC complex, MCAT, KAR, HAD, ENR, SAD and LPAAT. However, many genes involved in oil biosynthesis are sensitive to a high temperature environment and showed significant lower expression in the HT compare to the MT environment. The high temperature sensitive genes were ACC1, KASI, II and III, FATA and FATB, GPDH, GPAT, DGATs and PDAT. FAD2 and PDCT were also high temperature sensitive genes, but showed increased expression in HT compared to the MT environment. Oil accumulation was shown to be temperature sensitive and high temperatures resulted in decreased olive oil content in the mesocarp [17,36]. Conversion of acetyl-CoA to malonyl- CoA is catalyzed by ACC1 which is a key regulator of fatty acid synthesis. Overexpression of ACC1 in yeast significantly increased the total fatty acid content [49–51]. We found that OeACC1 (OE6A049983) is repressed in an HT environment. At 146 DPA, the ratio of the OeACC1 expression level between MT and HT environment was 2.9:1 in the heat tolerant ‘Barnea’ cultivar and 24:1 in the heat sensitive cultivar ‘Souri’. However, in the sensitive cultivar ‘Koroneiki’ the ratio was 4.1:1 and in the tolerant cultivars ‘Coratina’ and ‘Picholine’ the ratio was 9:1 and 4.8:1, respectively (Figure 3). Based on these results, we can conclude that OeACC1 is repressed at high temperatures. However, this gene cannot serve as a marker to discriminate between tolerant and sensitive cultivars in regard to heat stress. All three KAS genes were found to be sensitive to high temperatures and their expression was repressed in the HT environment. Characterizing only the gene with the highest expression from each gene family, we found that KASI (OE6A049543) was significantly overexpressed in the MT environment at all three time-points in the ‘Barnea’ cultivar, but only at 146 DPA in the ‘Souri’ cultivar. KASII (OE6A089647) was significantly overexpressed in the MT environment at all three time-points in the ‘Souri’ cultivar, but only at 83 and 104 DPA in the ‘Barnea’ cultivar. KASIII (OE6A034993) was significantly overexpressed in the MT environment at all three time-points in both cultivars. KAS genes, like OeACC1, are repressed in high temperatures. However, they also cannot serve as markers to discriminate between tolerant and sensitive cultivars to heat stress. Most of the genes involved in the fatty acid synthesis occurring in the ER were found to be HT regulated. GPDH, GPAT, DGAT1, DGAT2 and PDAT expression was repressed in HT environment. DGAT plays a key role in determining the carbon flux into TAG [52], and repression of its expression may lead to decreased oil accumulation as we found earlier [36]. However, FAD2 and PDCT were induced in under these conditions (Figure 2). Fatty acid modification from oleic acid to linoleic acid (C18:2) is catalyzed by FAD2 and FAD6 and from oleic acid to linolenic acid (C18:3) by FAD3 [32]. FAD3 genes were not expressed and the OeFAD6 gene was minimally expressed in both cultivars and in both environments. Among the FAD2 genes, OeFAD2-2 and OeFAD2-3 were also expressed at low levels in both cultivars and in both environments. OeFAD2-5, OeFAD2-1 and OeFAD2-4 were highly expressed in some samples. In the ‘Souri’ cultivar, OeFAD2-4 was expressed similarly in both environments, OeFAD2-1 expressed similarly in both environments at 104 and 146 DPA but induced in the HT environment at 83 DPA, OeFAD2-5 induced in the HT environment at 83 and 146 DPA. In the ‘Barnea’ cultivar, with the exception of OeFAD2-4, which was expressed similarly in both environments at 146 DPA, all three expressed FAD2 genes were induced in the HT environment at all sampled time-points. This is in agreement with other studies which found that FAD2 genes expression was induced by high temperatures [53]. However, other studies found that low temperatures also induced FAD2 genes in olives [54]. PDCT genes were induced in the HT environment in the ‘Souri’ cultivar, but not in the ‘Barnea’ cultivar. The PDCT enzyme uses the DAG as precursor and returns it to the PC pool, thus reducing the amount of DAG that continues to synthesis of TAG and repressing oil production. The main genes whose expression was cultivar specific regarding ‘Barnea’ and the ‘Souri’, are PDCT and FAD2. PDCT was induced in the HT environment only in the heat sensitive cultivar, ‘Souri’, and it’s activity repressed oil production in that environment. However, in the ‘Barnea’ cultivar, its expression was constant in both environments. FAD2 was highly induced in the HT environment in the heat sensitive cultivar, ‘Barnea’ and its’ effect was evident in the quality of the oil produced under high temperature conditions. It was only slightly induced under similar environmental conditions in the heat resistant cultivar, ‘Souri’. OeFAD2-1 expression level in all five cultivars in both environments (Figure 3), was found to be in relatively good agreement with the oil quality sensitivity of these cultivars [36]. The cultivars ‘Barnea’ and ‘Picholine’ were found to be the most heat sensitive in terms of oil quality and these cultivars showed the highest expression level of OeFAD2-1 in the HT environment. The ‘Souri’ cultivar was characterized as the most heat resistant of the five in terms of oil quality and showed no expression of OeFAD2-1 under HT conditions. However, the cultivars ‘Koroneiki’ and ‘Coratina’ showed oil quality sensitivity under high temperature conditions, with relatively low expression of OeFAD2-1. The ‘Koroneiki’ cultivar, although found to be heat sensitive in term of oil quality, showed similar expression levels of OeFAD2-1 in both environments. OeACC1 and OeFAD2-1 expression patterns in the ‘Souri’ and ‘Barnea’ cultivars as demonstrated in the RT-PCR experiment showed the same trend as in the RNA-seq results. However, the ratio between the expression level in HT and MT environments was slightly different. This ratio in OeACC1 was 1:2.5 and 1:2 in the ‘Barnea’ and ‘Souri’ cultivars respectively according to the RNA-seq results and 1:2.9 and 1:25 respectively as appeared in the RT-PCR results. For OeFAD2-1, this ratio was 2.7:1 and 1:1.5 in the ‘Barnea’ and ‘Souri’ cultivars respectively according to the RNA-seq results and 2.7:1 and 1:10 respectively according to the RT- PCR results.
4.3. WRI1 and Dof4 Are the Main Transcription Factors Regulating Olive Oil Biosynthesis Pathway
Network analysis of the transcription factors and oil biosynthesis genes reveals transcription factors hubs, which hypothetically could regulate the genes involved in fatty acid biosynthesis in response to high temperatures. We found that among the transcription factors known to regulate oil biosynthesis, the transcription factors OeDof4.3, OeWRI1.1, OeDof4.4 and OeWRI1.2 were co- expressed with a high number of oil biosynthesis genes. The LEC1, L1L, MYB73 and Dof11 transcription factors were co-expressed with a relatively low number of genes involved in oil biosynthesis, suggesting that these TFs are not the main factors affecting the response of oil production to high temperatures. WRI1 was already suggested as the main regulator in transcriptional control of plant oil biosynthesis [35]. However, in order to validate the role of the suggested hub, more studies need to be carried out, including identifying the binding motifs of the transcription factors in the target gene promoters as well as chromatin immunoprecipitation experiments to show that indeed the suggested transcription factors interact with the promotor genes involved in oil biosynthesis.
5. Conclusions
Our study demonstrates the negative effect of a high temperature environment on several key genes critical to the production and quality of olive oil. Different olive cultivars have developed a variety of mechanisms to deal with different aspects of high temperature damage. In order to elucidate the mechanism of high temperature damage to olive oil, we characterized the expression pattern of genes involved in the olive oil biosynthesis pathway in a high temperature (HT) environment compared to expression patterns under moderate conditions (MT). We found that most of the genes regulated by high temperatures are common to different stages of fruit development. However, many of them are cultivar dependent. We hypothesize that a strong induction of heat- shock proteins, especially during the late stage of fruit development, can indicate heat tolerance. Many genes involved in the olive oil biosynthesis are down-regulated as a response to high temperatures. OePDCT as well as OeFAD2 genes showed cultivar dependent expression patterns strongly related to their heat tolerance. Hence, these genes are recommended as markers for screening of various cultivar to test their tolerance level for high summer temperatures. We also found that the transcription factors OeDof4.3, OeWRI1.1, OeDof4.4 and OeWRI1.2 were co-expressed with a high number of olive oil biosynthesis genes and therefore seem to be key factors in regulating the oil biosynthesis pathway in response to heat stress. Due to climate changes in recent years and the forecast for the future, the mechanisms of the various olive cultivars response to heat stress should be characterized in detail in order to identify existing cultivars or to develop new ones tolerant to high summer temperatures. These will hopefully produce high yields as well as quality oil in a changing environment.
Supplementary Materials: The following are available online at www.mdpi.com/2223-7747/9/9/1135/s1, Figure S1: Venn diagrams of the significantly regulated genes. Comparison of the regulated gene identities between the different sampled time points in each cultivar and environment (a) and between the two cultivars in each time point and environment (b), Figure S2: OeHSP70 expression analyzed by RT-PCR at 146 DPA, Table S1: Primers used in the real-time quantitative PCR, Table S2: Sequencing results. The left column describe the number of Days Post Anthesis (DPA). #raw-reads are the average number of reads for sequencing lane. #clean-reads are the average number of reads after filtering and % mapping is the percentage of reads mapped to the olive genome.
Author Contributions: The original design of the study was set up by G.B.-A., Z.K. and B.A.; Y.N. and M.S. performed all the molecular analyses; Y.N., M.S., I.B., Y.M. and G.B.-A. performed the field experiment; A.D.-F., R.H. and G.B.-A. performed the data analysis; G.B.-A. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding: This work was supported by a grant (No. 203-1170) of the Israeli Ministry of Agriculture and Rural Development.
Acknowledgments: We thank Yehuda Ben-Ari for valuable assistance in writing and editing this paper. Conflicts of Interest: The authors declare no conflict of interest.
Contenido realizado por:
Giora Ben Ari, Ph.D. (Researcher)
Manager de ARTOLIO ENI CBC MED
Olive pollination. Biochemical and anatomical characterization of the olive abscission zone in fruits and leaves. Olive breeding program. The effects of climate change on olive productivity. Argan oil. Profitable and Sustainable artisanal olive oil industry in the Mediterranean (ARTOLIO) - Fund by ENI CBC MED - http://www.enicbcmed.eu/projects/artolio
References
- Maestri, E.; Klueva, N.; Perrotta, C.; Gulli, M.; Nguyen, H.T.; Marmiroli, N. Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol. Biol. 2002, 48, 667–681, doi:10.1023/a:1014826730024.
- Wilhelm, E.P.; Mullen, R.; Keeling, P.; Singletary, G. Heat stress during grain filling in maize: Effects onkernel growth and metabolism. Crop Sci. 1999, 39, 1733–1741, doi:10.2135/cropsci1999.3961733x.
- Barnabas, B.; Jager, K.; Feher, A. The effect of drought and heat stress on reproductive processes in cereals.
Plant Cell Environ. 2008, 31, 11–38, doi:10.1111/j.1365-3040.2007.01727.x.
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273–273, doi:10.3389/fpls.2013.00273.
- Bray, E.A.; Bailey-Serres, J.; Weretilnyk, E. Biochemistry and Molecular Biology of Plants, Responses to Abiotic Stresses; Gruissem, W., Jones, R., Eds.; American Society of Plant Biologists: Rockville, MD, USA, 2000; pp. 1158–1203.
- Guy, C. Molecular responses of plants to cold shock and cold acclimation. J. Mol. Microbiol. Biotechnol. 1999, 1, 231–242.
- Weis, E.; Berry, J.A. Plants and high temperature stress. Symp. Soc. Exp. Biol. 1988, 42, 329–346.
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options.Front. Plant Sci. 2017, 8, doi:10.3389/fpls.2017.01147.
- Camejo, D.; Rodríguez, P.; Angeles Morales, M.; Miguel Dell’Amico, J.; Torrecillas, A.; Alarcón, J.J. Hightemperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J.
Plant Physiol. 2005, 162, 281–289, doi:10.1016/j.jplph.2004.07.014.
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Tanveer, M.; Ihsan, M.Z.; Shah, A.N.; Ullah, A.; Nasrullah; Khan,F.; et al. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol. Biochem. 2016, 103, 191–198, doi:10.1016/j.plaphy.2016.03.001.
- Ferris, R.; Ellis, R.H.; Wheeler, T.R.; Hadley, P. Effect of high temperature stress at anthesis on grain yield and biomass of field-grown crops of wheat. Ann. Bot. 1998, 82, 631–639, doi:10.1006/anbo.1998.0740.
- Rainey, K.M.; Griffiths, P.D. Evaluation of Phaseolus acutifolius A. Gray plant introductions under high temperatures in a controlled environment. Genet. Resour. Crop Evol. 2005, 52, 117–120, doi:10.1007/s10722- 004-1811-2.
- Vara Prasad, P.V.; Craufurd, P.Q.; Summerfield, R.J. Fruit Number in Relation to Pollen Production and Viability in Groundnut Exposed to Short Episodes of Heat Stress. Ann. Bot. 1999, 84, 381–386, doi:10.1006/anbo.1999.0926.
- Bellincontro, A.; Caruso, G.; Mencarelli, F.; Gucci, R. Oil accumulation in intact olive fruits measured by near infrared spectroscopy-acousto-optically tunable filter. J. Sci. Food Agric. 2013, 93, 1259–1265, doi:10.1002/jsfa.5899.
- Conde, C.; Delrot, S.; Gerós, H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J. Plant Physiol. 2008, 165, 1545–1562, doi:10.1016/j.jplph.2008.04.018.
- Servili, M. Olive oil processing technologies and investments. In Present and Future of the Mediterranean Olive Sector; Caballero, J., D’Andria, R., Fernández, M., Garrido, A., Rallo, L., Arroyo López, F.N., Arcas, N., Fernandez Escobar, R., López-Miranda, J., Msallem, M., et al., Eds.; CIHEAM IOC: Zaragoza, Spain, 2013; Volume 106, pp. 55–66.
- García-Inza, G.P.; Castro, D.N.; Hall, A.J.; Rousseaux, M.C. Responses to temperature of fruit dry weight, oil concentration, and oil fatty acid composition in olive (Olea europaea L. var. ‘Arauco’). Eur. J. Agron. 2014, 54, 107–115, doi:10.1016/j.eja.2013.12.005.
- Miserere, A.; Searles, P.S.; García-Inza, G.P.; Rousseaux, M.C. Elevated temperature affects vegetative growth and fruit oil concentration in olive trees (Olea europaea). Acta Hortic. 2018, 1199, 523–528.
- Rondanini, D.P.; Castro, D.N.; Searles, P.S.; Rousseaux, M.C. Contrasting patterns of fatty acid composition and oil accumulation during fruit growth in several olive varieties and locations in a non-Mediterranean region. Eur. J. Agron. 2014, 52, 237–246, doi:10.1016/j.eja.2013.09.002.
- Trentacoste, E.R.; Puertas, C.M.; Sadras, V.O. Modelling the intraspecific variation in the dynamics of fruit growth, oil and water concentration in olive (Olea europaea L.). Eur. J. Agron. 2012, 38, 83–93, doi:10.1016/j.eja.2012.01.001.
- García-Inza, G.P.; Castro, D.N.; Hall, A.J.; Rousseaux, M.C. Opposite oleic acid responses to temperature in oils from the seed and mesocarp of the olive fruit. Eur. J. Agron. 2016, 76, 138–147, doi:10.1016/j.eja.2016.03.003.
- García-Inza, G.P.; Hall, A.J.; Rousseaux, M.C. Proportion of oleic acid in olive oil as influenced by the dimensions of the daily temperature oscillation. Sci. Hortic. 2018, 227, 305–312, doi:10.1016/j.scienta.2017.09.030.
- Lombardo, N.; Marone, E.; Alessandrino, M.; Godino, G.; Madeo, A.; Fiorino, P. Influence of growing season temperatures in the fatty acids (FAs) of triacilglycerols (TAGs) composition in Italian cultivars of Olea europaea. Adv. Hortic. Sci. 2008, 22, 49–53.
- Mailer, R.J.; Ayton, J.; Graham, K. The Influence of Growing Region, Cultivar and Harvest Timing on the Diversity of Australian Olive Oil. J. Am. Oil Chem. Soc. 2010, 87, 877–884, doi:doi:10.1007/s11746-010-1608- 8.
- Al-Whaibi, M.H. Plant heat-shock proteins: A mini review. J. King Saud Univ. Sci. 2011, 23, 139–150, doi:10.1016/j.jksus.2010.06.022.
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.-X.; Zhang, H.-X.; Wei, A.-M.; Gong, Z.-H. Heat shock proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 5321, doi:10.3390/ijms20215321.
- Janni, M.; Gullì, M.; Maestri, E.; Marmiroli, M.; Valliyodan, B.; Nguyen, H.T.; Marmiroli, N. Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J. Exp. Bot. 2020, 71, 3780–3802, doi:10.1093/jxb/eraa034.
- Montero-Barrientos, M.; Hermosa, R.; Nicolás, C.; Cardoza, R.E.; Gutiérrez, S.; Monte, E. Overexpression of a Trichoderma HSP70 gene increases fungal resistance to heat and other abiotic stresses. Fungal Genet. Biol. 2008, 45, 1506–1513, doi:10.1016/j.fgb.2008.09.003.
- Qi, Y.; Wang, H.; Zou, Y.; Liu, C.; Liu, Y.; Wang, Y.; Zhang, W. Over-expression of mitochondrial heat shock protein 70 suppresses programmed cell death in rice. FEBS Lett. 2011, 585, 231–239, doi:10.1016/j.febslet.2010.11.051.
- Wang, X.; Yan, B.; Shi, M.; Zhou, W.; Zekria, D.; Wang, H.; Kai, G. Overexpression of a Brassica campestris HSP70 in tobacco confers enhanced tolerance to heat stress. Protoplasma 2016, 253, 637–645, doi:10.1007/s00709-015-0867-5.
- He, M.; Qin, C.-X.; Wang, X.; Ding, N.-Z. Plant unsaturated fatty acids: Biosynthesis and regulation. Front. Plant Sci. 2020, 11, doi:10.3389/fpls.2020.00390.
- Salas, J.; Harwood, J.; Martínez Force, E. Lipid metabolism in olive: Biosynthesis of triacylglycerols and aroma components. In Handbook of Olive Oil, 2nd ed.; Aparicio, R., Harwood, J., Eds.; Springer: New York, NY, USA, 2013, doi:10.1007/978-1-4614-7777-8_4pp. 97-127.
- Hu, Z.; Ren, Z.; Lu, C. The phosphatidylcholine diacylglycerol cholinephosphotransferase is required for efficient hydroxy fatty acid accumulation in transgenic Arabidopsis. Plant Physiol. 2012, 158, 1944–1954, doi:10.1104/pp.111.192153.
- Manan, S.; Chen, B.; She, G.; Wan, X.; Zhao, J. Transport and transcriptional regulation of oil production in plants. Crit. Rev. Biotechnol. 2017, 37, 641–655, doi:10.1080/07388551.2016.1212185.
- Kong, Q.; Yuan, L.; Ma, W. WRINKLED1, a “Master Regulator” in transcriptional control of plant oil biosynthesis. Plants 2019, 8, 238, doi:10.3390/plants8070238.
- Nissim, Y.; Shloberg, M.; Biton, I.; Many, Y.; Doron-Faigenboim, A.; Zemach, H.; Hovav, R.; Kerem, Z.; Avidan, B.; Ben-Ari, G. High temperature environment reduces olive oil yield and quality. PLoS ONE 2020, 15, e0231956, doi:10.1371/journal.pone.0231956.
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652.
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, doi:10.1093/bioinformatics/btu170.
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25, doi:10.1186/gb-2009-10-3-r25.
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323, doi:10.1186/1471-2105-12-323.
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386.
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140, doi:10.1093/bioinformatics/btp616.
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300.
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80, doi:10.1186/gb-2004-5-10-r80.
- SAS Institute JMP® Version 7 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2007.
- Cruz, F.; Julca, I.; Gómez-Garrido, J.; Loska, D.; Marcet-Houben, M.; Cano, E.; Galán, B.; Frias, L.; Ribeca, P.; Derdak, S.; et al. Genome sequence of the olive tree, Olea europaea. GigaScience 2016, 5, 29 doi:10.1186/s13742-016-0134-5.
- Huang, L.-J.; Cheng, G.-X.; Khan, A.; Wei, A.-M.; Yu, Q.-H.; Yang, S.-B.; Luo, D.-X.; Gong, Z.-H. CaHSP16.4, a small heat shock protein gene in pepper, is involved in heat and drought tolerance. Protoplasma 2019, 256,39–51, doi:10.1007/s00709-018-1280-7.
- Xu, Y.; Zhan, C.; Huang, B. Heat shock proteins in association with heat tolerance in grasses. Int. J. Proteom. 2011, 2011, 529648, doi:10.1155/2011/529648.
- Fernandez-Moya, R.; Da Silva, N.A. Engineering Saccharomyces cerevisiae for high-level synthesis of fatty acids and derived products. FEMS Yeast Res. 2017, 17, doi:10.1093/femsyr/fox071.
- Gomma, A.E.; Lee, S.-K.; Sun, S.M.; Yang, S.H.; Chung, G. Improvement in oil production by increasing Malonyl-CoA and Glycerol-3-Phosphate pools in scenedesmus quadricauda. Indian J. Microbiol. 2015, 55, 447–455, doi:10.1007/s12088-015-0546-4.
- Shin, G.-H.; Veen, M.; Stahl, U.; Lang, C. Overexpression of genes of the fatty acid biosynthetic pathway leads to accumulation of sterols in Saccharomyces cerevisiae. Yeast 2012, 29, 371–383, doi:10.1002/yea.2916.
- Liu, Q.; Siloto, R.M.P.; Lehner, R.; Stone, S.J.; Weselake, R.J. Acyl-CoA:diacylglycerol acyltransferase: Molecular biology, biochemistry and biotechnology. Prog. Lipid Res. 2012, 51, 350–377, doi:10.1016/j.plipres.2012.06.001.
- Li, Q.; Zheng, Q.; Shen, W.; Cram, D.; Fowler, D.B.; Wei, Y.; Zou, J. Understanding the biochemical basis of temperature-Induced lipid pathway adjustments in plants. Plant Cell 2015, 27, 86–103, doi:10.1105/tpc.114.134338.
- Luisa Hernández, M.; Dolores Sicardo, M.; Arjona, P.M.; Martínez-Rivas, J.M. Specialized functions of olive FAD2 gene family members related to fruit development and the abiotic stress response. Plant Cell Physiol. 2019, 61, 427–441, doi:10.1093/pcp/pcz208.© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Unlocking engagement success: a complete guide to creating appealing social media content.
Unlocking engagement success: a complete guide to creating appealing social media content.
Introduction to the development of attractive content for social networks
Creating engaging content for social media is critical to any digital marketing strategy. Millions of people use these platforms daily. This is why it is essential to learn how to create social media content that attracts your audience and generates engagement. In this article, we will explore the importance of social media engagement and provide you with a comprehensive guide to developing engaging content.
Understanding engagement and its importance in social networks.
Engagement is the interaction that takes place between users of a social media platform and the content they share. This may include “likes”, comments, sharing and other types of interactions. The higher the engagement, the more likely your content will be distributed across the platform and reach more people.
Engagement is important in social media because it helps increase your brand visibility, foster strong relationships with your followers and drive traffic to your website. A high level of engagement can also improve your online reputation and generate more business opportunities.

How to create content for social networks: a brief description
There are several strategies and approaches you can use to create engaging content on social networks. Some of these include:
- Share relevant and valuable content that resonates with your audience
- Use high-quality images and videos to capture the users' attention
- Publish updates about the industry and news to keep your audience informed
- Participate in online conversations and discussions to encourage interaction and engagement
- Use relevant hashtags and keywords to increase the visibility of your content
In the following sections, we will explore these approaches in detail and provide you with an 8-step process to generate engagement on social media platforms.
8 steps to generate engagement on social media platforms
By following these 8 steps, you will be able to create interesting content that will bring forth engagement on social networks and increase your brand's visibility.
- Define objectives and goals: Before you start creating content, it is important to set clear objectives and goals for your social media strategy. These may include increasing brand visibility, driving traffic to your website or generating sales. Having specific objectives will help you create content that is aligned with your goals and measure your success.
- Know your audience: To create content that resonates with your audience, you must know their interests, needs and preferences. Research and analyze your audience to understand their behaviors, demographics and content consumption habits. This will allow you to create content that is relevant and valuable to them.
- Create a content calendar: A content calendar is a planning tool that helps you organize and schedule your social media posts. By creating a content calendar, you can ensure that your content is evenly distributed and published at optimal times to increase engagement.
- Produce quality content: The quality of the content you share on social networks is fundamental to creating engagement. Make sure your content is relevant, valuable and visually appealing. Use high-quality images and videos, write engaging copy, and use a tone and writing style that resonates with your audience.
- Optimize your content for each platform: Each social media platform has its own characteristics and best practices. Tailor your content for each platform by making sure it fits the right formats and dimensions, and use relevant hashtags and keywords to increase your visibility.
- Encourage interaction and engagement: To generate engagement on social networks, you must be willing to interact with your audience and encourage online conversations. Respond to comments and questions from your followers, engage in debates and discussions, and share user-generated content to strengthen relationships with your audience.
- Monitor and measure the success of your content: Use analytics tools to monitor the performance of your content on social networks and evaluate the success of your strategy. Pay attention to engagement metrics such as “likes”; comments, shares and clicks, and use this data to improve your content and adjust your strategy accordingly.
- Refine and improve your strategy: Based on the data and feedback you collect, refine and improve your social media content strategy. Make sure you are always aware of trends and changes in social media platforms and adapt your approach accordingly.
How to develop content for different social media platforms
Each social media platform has its own characteristics, formats and best practices. Here are some tips for developing engaging content and generating engagement on the major platforms:
- Use high-quality images and videos to capture users' attention.
- Share links to articles and blog posts relevant to your audience.
- Use a conversational and friendly writing tone.
- Ask questions and encourage followers to share their opinions and experiences.
- Take advantage of Facebook's interactive features, such as polls and live broadcasts.
- Share visually appealing, high-quality images and videos.
- Use relevant hashtags to increase the visibility of your content.
- Share Instagram stories to provide a more personal and authentic view of your brand.
- Collaborate with influencers and other related accounts to increase your reach and engagement.
- Take advantage of Instagram's interactive features, such as question stickers and custom filters.
- Share industry updates and news to keep your audience informed.
- Use a concise and direct writing tone.
- Participate in online conversations and discussions using relevant hashtags.
- Respond to your followers' mentions and questions to encourage interaction and engagement.
- Share user-generated content and retweet other users' posts related to your brand.
- Share professional, industry-oriented content such as articles, case studies and blog posts.
- Use a more formal and professional writing tone.
- Participate in discussion groups and forums related to your industry.
- Share company updates and achievements to highlight your brand's culture and values.
- Leverage LinkedIn's publishing features to share long-form content and generate engagement.
Tips and tricks for maintaining a consistent brand voice
Maintaining a consistent brand voice on social media is essential to generating engagement and building a strong brand identity. Here are some tips and tricks to achieve this:
- Establish style guidelines: Create style guidelines that include details about your writing tone, use of keywords and phrases, and content formatting. These guidelines will help ensure that your content is consistent and aligned with your brand's personality.
- Tailor your voice to each platform: While it's important to maintain a consistent brand voice, you should also adapt your tone and writing style to each social media platform. For example, you can adopt a more informal and friendly tone on Facebook, while on LinkedIn, you may want to use a more formal and professional tone.
- Train your team: Make sure everyone on your team is familiar with your brand's style guidelines and knows how to apply them to social media content. Provide training and feedback to ensure your team is aligned with your brand voice and style.
- Use collaboration tools: Use collaboration tools, such as Trello or Asana, to share and review content on social networks before publishing it. This will ensure that your content is consistent and aligned with your brand voice.
- Monitor and adjust your brand voice: Be aware of feedback from your audience and use this data to adjust and improve your brand voice on social media. Always be willing to adapt and evolve according to the needs and preferences of your audience.
Using analytics to measure and optimize the success of your content
Social media analytics is essential to measure the success of your content and optimize your strategy. Here are some key metrics to monitor:
- Reach: The number of people who see your content on social networks.
- Impressions: The number of times your content is shown on social networks, regardless of whether it is clicked on or not.
- Engagement: The number of interactions your content receives, including likes, comments and shares.
- Clicks: The number of times users click on links or interactive elements in your content.
- Conversions: The number of users who take a specific action after interacting with your content, such as subscribing to your newsletter or making a purchase.
Use analytics tools, such as Google Analytics and native social media analytics platforms, to monitor these metrics and evaluate the success of your content strategy. Use this data to adjust and improve your approach and optimize the performance of your social media content.
Examples of successful content and engagement strategies in social networks.
There are numerous examples of brands that have achieved great success on social media through effective content and engagement strategies. Some of these include:
- Nike: Nike uses high-quality images and videos in its social media posts to capture the attention of its audience. They also leverage the power of influencers and sponsored athletes to increase their reach and generate engagement.
- Coca-Cola: Coca-Cola has adopted a conversational and friendly writing tone in their social networks, which allows them to interact effectively with their audience. They also focus on sharing user-generated content and fostering online conversations about their brand.
- Airbnb: Airbnb uses social media to tell stories about their hosts and guests, allowing them to showcase their brand in an authentic and personal way. They also actively participate in online conversations and respond quickly to questions and comments from their followers.
Study these and other successful examples to get ideas and inspiration for your own social media content strategy.
Tools and resources to simplify the management of your social networks
In addition to analytics tools, there are numerous tools and resources you can use to simplify the management and optimization of your social networks. These include scheduling tools, such as Hootsuite and Sprout Social, that allow you to schedule posts on multiple platforms at the same time. You can also find free content templates, step-by-step guides for creating engaging content and tools for measuring performance.
Research these resources and find the ones that best suit your needs. Set a budget for the management of your social networks and find the best way to achieve your business objectives. Make sure your strategy is in line with your business objectives and scalable as your online presence grows. Finally, maintain constant communication with your audience and continue to optimize your efforts to ensure the best possible performance.
Content created by:
Jesus F. Gordillo
Communication Manager of ARTOLIO ENI CBC MED
Strategic Director of Kellenföl Ad, an agency based in Barcelona. Advisor to companies and public institutions. Professor of marketing and advertising strategies.
Unleashing the power of agricultural marketing: Essential strategies for modern farmers.
Unleashing the power of agricultural marketing: Essential strategies for modern farmers.
Introduction to agricultural marketing
Agricultural marketing, also known as agromarketing, is a crucial component to the success of any farmer's brand nowadays. Globalization and increasing competition in the agricultural sector have made marketing an essential element in building and maintaining a strong customer base and ensuring the sustainability of an agricultural business. Agromarketing is a holistic approach that includes the planning, implementation and control of all activities related to the promotion, sale and distribution of agricultural products.
Agriculture has undergone a significant transformation in recent decades, and technological advances have changed the way farmers produce, process and market their products. Agromarketing is a powerful tool that helps farmers adapt to these new realities and make the most of market opportunities. In this article, we will discuss the essential strategies that modern farmers should embrace to take full advantage of the power of agricultural marketing.
The importance of agromarketing in modern agriculture
Nowadays, agromarketing is critical to the success of any agribusiness. Increasing competition and the growing demand for high-quality agricultural products make marketing more important than ever. Growers must be aware of market trends, consumer needs and wants, and the practices of their competitors to stay on top of the industry and maintain a competitive advantage.
Agromarketing is also important to ensure the sustainability of an agricultural business. Farmers who adopt effective marketing practices can establish strong and lasting relationships with their customers and other key players in the supply chain. This allows them to maintain a steady revenue stream and ensure the long-term financial viability of their businesses.
In addition, agromarketing helps farmers communicate the value of their products to consumers. This is especially relevant in a world where consumers are increasingly concerned about the quality, safety, and sustainability of the agricultural products they buy. Farmers who can effectively communicate their ethical and sustainable practices and the quality of their products have a competitive advantage in the marketplace and can obtain higher prices for their products.
 Key principles of agricultural marketing
Key principles of agricultural marketing
Agricultural marketing is a holistic approach that involves several key principles that farmers should keep in mind when developing and implementing their marketing strategies. These principles include:
- Customer orientation: Farmers should focus on meeting the needs and desires of their customers, and adapt their products and practices accordingly. This involves researching and understanding customer preferences and tailoring the products and services offered to meet those preferences.
- Market segmentation: Farmers must identify and target specific market segments that are more likely to buy their products. This involves analyzing the market based on demographic, geographic, psychographic and behavioral factors and developing products and marketing strategies that fit the needs and desires of each segment.
- Differentiation and positioning: Farmers must differentiate their products and services from those of their competitors and position them uniquely in the customer's mind. This involves effectively communicating the benefits and unique features of the products and services offered, and establishing a strong and consistent brand image.
- Marketing mix: Farmers must employ an appropriate mix of the four “P 's” of marketing (product, price, promotion and place) to achieve their marketing objectives. This involves making decisions about product quality and features, pricing strategy, promotional tactics and the distribution channels to be used.
Understanding the market and the target audience
To develop an effective agricultural marketing strategy, farmers must first understand the market in which they operate and the target audience they are addressing. It involves researching and analyzing market trends, consumer needs and wants, and the practices of your competitors. Growers can obtain valuable market and target audience information through a variety of sources, such as market research reports, case studies, competitor analysis, and interviews with customers and industry experts.
By understanding the market and the target audience, farmers can identify opportunities and challenges that they can leverage to improve their operations and better position themselves in the market. They can also tailor their products and services to meet the needs and desires of consumers and differentiate themselves from their competitors.
Developing an effective agromarketing strategy
Once farmers have researched and understood the market and target audience, they can begin to develop an effective farm marketing strategy. This strategy should include clear and specific marketing objectives, as well as specific tactics and actions to achieve those objectives. Examples of agricultural marketing objectives may include increasing brand awareness, increasing sales, improving customer loyalty or entering new markets.
When developing an agromarketing strategy, farmers should consider the key agricultural marketing principles mentioned above, such as customer focus, market segmentation, differentiation and positioning, and marketing mix. They must also take into account external factors, such as economic conditions, market trends and government regulations, which may affect their business and marketing operations.
Using digital platforms for agricultural marketing
Digital platforms have changed the way farmers market their products and connect with their customers. Farmers can leverage a variety of digital platforms to reach their target audience effectively and efficiently. Some examples of digital platforms that farmers can use for agricultural marketing include:
- Websites and blogs: Farmers can create websites and blogs to share information about their products, farming practices and the latest news about the industry. This allows them to establish an online presence and increase their visibility in the market.
- Social media: Farmers can use social media platforms such as Facebook, Instagram, Twitter, and LinkedIn to connect with their customers, share relevant and engaging content and promote their products and services.
- E-commerce: Farmers can use e-commerce platforms to sell their products directly to consumers, allowing them to reach a wider audience and increase their income.
- Email marketing: Farmers can use email marketing to keep in touch with their customers and send them information about promotions, events and relevant news.
- Online advertising: Farmers can use online advertising, such as Google AdWords or Facebook Ads, to reach a specific target audience and promote their products and services.
Agromarketing tools and channels
In addition to digital platforms, farmers can also employ a variety of traditional marketing tools and channels to promote their products and services. Examples of agromarketing tools and channels include:
- Agricultural fairs and exhibitions: Farmers can participate in agricultural fairs and exhibitions to showcase their products and connect with customers and other key players in the industry.
- Public relations and press releases: Farmers can use public relations and press releases to communicate important news and information about their products and farming practices to the media and the public.
- Printed promotional materials: Farmers can use printed promotional materials, such as brochures, catalogs and business cards, to share information about their products and services with customers and prospects.
- Traditional media advertising: Farmers can use traditional media advertising, such as newspaper, magazine, radio and television advertisements, to reach a wider audience and promote their products and services.
Building long-term relationships with customers and stakeholders
Building long-term relationships with customers and other key players in the supply chain is essential to the success of any agricultural business. Farmers who are able to establish strong and lasting relationships with their customers and partners are more likely to maintain a steady stream of income and ensure the long-term financial viability of their businesses.
To build long-term relationships, growers must focus on customer satisfaction and providing exceptional customer service. This involves listening and responding to customers' needs and desires, solving problems quickly and effectively, and communicating openly and transparently with customers. Farmers can also use customer loyalty programs and offer incentives for repeat purchases, such as discounts and special offers.
In addition to customers, farmers must also establish strong relationships with other key players in the supply chain, such as suppliers, distributors and regulators. This involves establishing trusting and collaborative relationships and working together to improve the quality and efficiency of supply chain operations.
Measuring agromarketing success
It is important for farmers to measure and evaluate the success of their farm marketing efforts to determine which strategies and tactics are working and which areas need improvement. Some metrics that farmers can use to measure agromarketing success include:
- Return on Investment (ROI): Farmers can calculate their ROI to evaluate the effectiveness of their marketing efforts and determine if they are getting an adequate return on their investment.
- Conversion rate: Farmers can measure the conversion rate of potential customers to actual customers to determine the effectiveness of their marketing tactics.
- Online engagement: Farmers can measure online engagement, such as the number of social media followers and website traffic, to evaluate the effectiveness of their online marketing efforts.
- Customer satisfaction surveys: Farmers can conduct customer satisfaction surveys to assess customer satisfaction and determine areas for improvement.
The best resources for agricultural marketing education and training
There is a wide variety of resources and training programs available for farmers looking to strengthen their agricultural marketing skills and knowledge. Some of the best resources
Content created by:
Jesus F. Gordillo
Communication Manager of ARTOLIO ENI CBC MED
Strategic Director of Kellenföl Ad, an agency based in Barcelona. Advisor to companies and public institutions. Professor of marketing and advertising strategies.
Empowering small producers: Artolio Eni CBC Med's impact on the Mediterranean extra virgin olive oil industry
Empowering small producers: Artolio Eni CBC Med's impact on the Mediterranean extra virgin olive oil industry
Introduction to the Mediterranean Extra Virgin Olive Oil Industry
Extra virgin olive oil is one of the most representative products of the Mediterranean diet. Its production and consumption are closely linked to the history, culture and economy of Mediterranean countries. The quality of extra virgin olive oil is fundamental to guaranteeing its healthy and organoleptic properties, and for this reason, the sector is subject to rigorous quality controls and designations of origin.
Currently, the global extra virgin olive oil market is dominated by large companies that can afford the costs of production and distribution, leaving small producers at a disadvantage. The latter often lack the necessary resources to compete, which makes it difficult for them to stay in business and preserve the traditions and production techniques that guarantee product quality.
Challenges facing small-scale producers in the sector
Small producers of extra virgin olive oil face numerous challenges to stay in the market. One of the main problems is the lack of access to adequate financing to invest in technology and improve their production processes. In addition, they tend to have fewer resources to promote their products and create a recognized brand in the national and international market.
Globalization and price competition also represent important challenges for small producers. Access to international markets can be complicated due to trade barriers and transportation and distribution costs. In addition, competition from other extra virgin olive oil producing countries, which can offer lower prices, hinders the profitability of small producers.
 Artolio Eni CBC Med's mission to empower smallholder farmers
Artolio Eni CBC Med's mission to empower smallholder farmers
Artolio Eni CBC Med is a project that seeks to empower small-scale extra virgin olive oil producers in the Mediterranean through the promotion of sustainable practices and the strengthening of their productive and commercial capacities. The main objective of this project is to improve the competitiveness and added value of small-scale producers, while preserving the environment and cultural traditions.
Artolio Eni CBC Med works in collaboration with local and international organizations to develop tools and strategies to facilitate the adoption of sustainable practices in the extra virgin olive oil industry. In addition, the project seeks to facilitate access to financing and international markets for small producers, as well as to promote cooperation among them to share knowledge and resources.
Artolio Eni CBC Med Success Stories
Thanks to Artolio Eni CBC Med's support, numerous small Mediterranean extra virgin olive oil producers have been able to improve their production practices and increase their competitiveness on the market. These success stories demonstrate the potential of this project to transform the industry and generate economic, social and environmental benefits for local communities.
One of the success stories is that of a small producer in Greece who managed to obtain organic certification for his extra virgin olive oil, which allowed him to access new markets and improve his income. Another case is that of a group producers who, thanks to technical and financial support from Artolio Eni CBC Med, were able to modernize their facilities and improve the quality of their product.
Sustainable practices in Mediterranean extra virgin olive oil production
One of the pillars of Artolio Eni CBC Med is the promotion of sustainable practices in the production of extra virgin olive oil. These practices include the use of environmentally friendly agricultural techniques, such as organic farming, the responsible use of water resources and the reduction in the use of chemical products. In addition, the project encourages the adoption of technologies that improve energy efficiency and reduce greenhouse gas emissions.
Sustainability also applies to the management of by-products and waste generated during the production process. Artolio Eni CBC Med promotes the reuse and recycling of these by-products, as well as the creation of new value-added products from them, such as fertilizers or biomass for energy production.
Economic impact of Artolio Eni CBC Med on the industry
Artolio Eni CBC Med's support to small Mediterranean extra virgin olive oil producers has generated a significant economic impact on the industry. By improving the competitiveness and added value of these producers, new jobs have been created and sources of income for local communities have been diversified.
In addition, the strengthening of the productive and commercial capacities of small producers has made it possible to increase exports of extra virgin olive oil from the Mediterranean, thus helping to improve the trade balance of the countries involved in the project.
Social and environmental benefits of support to small producers
Supporting small Mediterranean extra virgin olive oil producers through the Artolio Eni CBC Med project generates significant social and environmental benefits. The promotion of sustainable practices contributes to environmental conservation and the fight against climate change, while preserving the cultural traditions and agricultural heritage of local communities.
In addition, by improving the quality of life and income of small producers, it promotes rural development and helps reduce migration to urban areas. This, in turn, favors social cohesion and the strengthening of community networks.
How consumers can contribute to the empowerment of small producers
Consumers have a key role to play in empowering small Mediterranean extra virgin olive oil producers. By choosing products made by these producers, the customer can support their economic activity and contribute to the preservation of the environment and the cultural heritage associated with the production of extra virgin olive oil.
In addition, consumers can promote the consumption of quality extra virgin olive oil of sustainable origin by disseminating information and supporting initiatives such as Artolio Eni CBC Med. This raises awareness of the importance of preserving quality and sustainability in the extra virgin olive oil industry.
Future outlook for the Mediterranean extra virgin olive oil industry
The future of the Mediterranean extra virgin olive oil industry depends to a large extent on the ability of small producers to meet the challenges posed by the global market. Projects such as Artolio Eni CBC Med are fundamental to guarantee the sustainability and competitiveness of these producers, and to preserve the quality and identity of extra virgin olive oil.
As the demand for healthy and sustainable products continues to increase, Mediterranean extra virgin olive oil is expected to maintain its privileged position in the global market. However, this requires continued investment in strengthening the productive and commercial capacities of small producers, as well as in promoting sustainable practices and technological innovation.
In addition, it is important that governments and international organizations continue to support the development of the Mediterranean extra virgin olive oil industry, ensuring a favorable economic and legal environment for small producers and promoting cooperation between the different actors in the sector.
Conclusions: The lasting impact of Artolio Eni CBC Med.
In conclusion, the Artolio Eni CBC Med project is having a significant impact on the Mediterranean extra virgin olive oil industry by empowering small producers and promoting sustainable practices and capacity building. Through their work, they are generating significant economic, social and environmental benefits for local communities.
However, much remains to be done to ensure the sustainability and competitiveness of the Mediterranean extra virgin olive oil industry in the future. It is necessary to continue supporting small producers and promoting sustainable practices and technological innovation, as well as fostering cooperation among the different actors in the sector.
As consumers, we can also contribute to the empowerment of small Mediterranean extra virgin olive oil producers through our purchasing decisions and the promotion of sustainable practices in the industry. Together, we can ensure a sustainable and prosperous future for this industry that is so important to the Mediterranean economy, culture and environment.
Content created by:
Jesus F. Gordillo
Communication Manager of ARTOLIO ENI CBC MED
Strategic Director of Kellenföl Ad, an agency based in Barcelona. Advisor to companies and public institutions. Professor of marketing and advertising strategies.










